Abstract
Background/Objectives:
Limited numbers of studies demonstrated obesity-induced macrophage infiltration in skeletal muscle (SM), but dynamics of immune cell accumulation and contribution of T cells to SM insulin resistance are understudied.
Subjects/Methods:
T cells and macrophage markers were examined in SM of obese humans by reverse transcription-PCR (RT-PCR). Mice were fed high-fat diet (HFD) for 2–24 weeks, and time course of macrophage and T-cell accumulation was assessed by flow cytometry and quantitative RT-PCR. Extramyocellular adipose tissue (EMAT) was quantified by high-resolution micro-computed tomography (CT), and correlation to T-cell number in SM was examined. CD11a–/– mice and C57BL/6 mice were treated with CD11a-neutralizing antibody to determine the role of CD11a in T-cell accumulation in SM. To investigate the involvement of Janus kinase/signal transducer and activator of transcription (JAK/STAT), the major pathway for T helper I (TH1) cytokine interferon-γ, in SM and adipose tissue inflammation and insulin resistance, mice were treated with a JAK1/JAK2 inhibitor, baricitinib.
Results:
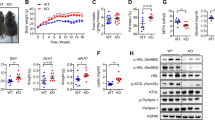
Macrophage and T-cell markers were upregulated in SM of obese compared with lean humans. SM of obese mice had higher expression of inflammatory cytokines, with macrophages increasing by 2 weeks on HFD and T cells increasing by 8 weeks. The immune cells were localized in EMAT. Micro-CT revealed that EMAT expansion in obese mice correlated with T-cell infiltration and insulin resistance. Deficiency or neutralization of CD11a reduced T-cell accumulation in SM of obese mice. T cells polarized into a proinflammatory TH1 phenotype, with increased STAT1 phosphorylation in SM of obese mice. In vivo inhibition of JAK/STAT pathway with baricitinib reduced T-cell numbers and activation markers in SM and adipose tissue and improved insulin resistance in obese mice.
Conclusions:
Obesity-induced expansion of EMAT in SM was associated with accumulation and proinflammatory polarization of T cells, which may regulate SM metabolic functions through paracrine mechanisms. Obesity-associated SM ‘adiposopathy' may thus have an important role in the development of insulin resistance and inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Olefsky JM, Glass CK . Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246.
Plomgaard P, Nielsen AR, Fischer CP, Mortensen OH, Broholm C, Penkowa M et al. Associations between insulin resistance and TNF-alpha in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia 2007; 50: 2562–2571.
Lumeng CN, Bodzin JL, Saltiel AR . Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184.
Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH et al. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol 2009; 30: 186–192.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830.
Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A . Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem 1993; 268: 26055–26058.
Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007; 115: 1029–1038.
Rausch ME, Weisberg S, Vardhana P, Tortoriello DV . Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008; 32: 451–463.
Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008; 28: 1304–1310.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914–920.
Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab 2013; 17: 411–422.
Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS . T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010; 18: 1918–1925.
Jiang E, Perrard XD, Yang D, Khan IM, Perrard JL, Smith CW et al. Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissuue. Arterioscler Thromb Vasc Biol 2014; 34: 34–43.
Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15: 930–939.
Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009; 15: 921–929.
McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM et al. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem 2009; 284: 31936–31944.
Xu H, Sethi JK, Hotamisligil GS . Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J Biol Chem 1999; 274: 26287–26295.
Souza SC, Palmer HJ, Kang YH, Yamamoto MT, Muliro KV, Paulson KE et al. TNF-alpha induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. J Cell Biochem 2003; 89: 1077–1086.
Guilherme A, Virbasius JV, Puri V, Czech MP . Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9: 367–377.
Pillon NJ, Bilan PJ, Fink LN, Klip A . Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab 2013; 304: E453–E465.
DeFronzo RA, Tripathy D . Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009; 32: S157–S163.
Ryder JW, Gilbert M, Zierath JR . Skeletal muscle and insulin sensitivity: pathophysiological alterations. Front Biosci 2001; 6: D154–D163.
Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 1997; 46: 983–988.
Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW . Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 1991; 40: 280–289.
Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE . Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997; 46: 1579–1585.
Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 1999; 42: 113–116.
Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 1999; 48: 1600–1606.
Moro C, Bajpeyi S, Smith SR . Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 2008; 294: E203–E213.
Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ et al. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 2002; 51: 1022–1027.
Goodpaster BH, Thaete FL, Kelley DE . Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000; 71: 885–892.
Goss AM, Gower BA . Insulin sensitivity is associated with thigh adipose tissue distribution in healthy postmenopausal women. Metabolism 2012; 61: 1817–1823.
Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 2009; 296: E1300–E1310.
Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 2007; 117: 1658–1669.
Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG . Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 2008; 8: 301–309.
Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A et al. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) 2013; 22: 747–757.
Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 2009; 58: 2525–2535.
Bruun JM, Helge JW, Richelsen B, Stallknecht B . Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 2006; 290: E961–E967.
Tam CS, Sparks LM, Johannsen DL, Covington JD, Church TS, Ravussin E . Low macrophage accumulation in skeletal muscle of obese type 2 diabetics and elderly subjects. Obesity (Silver Spring) 2012; 20: 1530–1533.
Khan IM, Dai Perrard XY, Perrard JL, Mansoori A, Wayne Smith C, Wu H et al. Attenuated adipose tissue and skeletal muscle inflammation in obese mice with combined CD4+ and CD8+ T cell deficiency. Atherosclerosis 2014; 233: 419–428.
Wu H, Rodgers JR, Perrard XY, Perrard JL, Prince JE, Abe Y et al. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol 2004; 173: 297–306.
Zhang Y, Pilon G, Marette A, Baracos VE . Cytokines and endotoxin induce cytokine receptors in skeletal muscle. Am J Physiol Endocrinol Metab 2000; 279: E196–E205.
Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD . How cells respond to interferons. Annu Rev Biochem 1998; 67: 227–264.
Luu YK, Lublinsky S, Ozcivici E, Capilla E, Pessin JE, Rubin CT et al. In vivo quantification of subcutaneous and visceral adiposity by micro-computed tomography in a small animal model. Med Eng Phys 2009; 31: 34–41.
Fink LN, Oberbach A, Costford SR, Chan KL, Sams A, Bluher M et al. Expression of anti-inflammatory macrophage genes within skeletal muscle correlates with insulin sensitivity in human obesity and type 2 diabetes. Diabetologia 2013; 56: 1623–1628.
Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 2010; 184: 5298–5307.
Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007; 56: 2910–2918.
Gunn MD, Nelken NA, Liao X, Williams LT . Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol 1997; 158: 376–383.
Schall TJ, Bacon K, Toy KJ, Goeddel DV . Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 1990; 347: 669–671.
Ley K, Laudanna C, Cybulsky MI, Nourshargh S . Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7: 678–689.
Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 2008; 103: 467–476.
Schulz EG, Mariani L, Radbruch A, Hofer T . Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity 2009; 30: 673–683.
Sica A, Mantovani A . Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122: 787–795.
Acknowledgements
This work was supported by NIH grants T32 GM88129 and T32 HL007812 (to IK), R01 HL098839 (to HW), P30 AI36211 (to DEL) and R01 DK078847 (to CMB). The authors thank Jerry L Perrard (Baylor College of Medicine) and Joshua Smith (Florida Hospital) for technical assistance, Ching H Tung, PhD (Houston Methodist Research Institute) for help with the CT imaging, Willa Hsueh, MD (Houston Methodist Research Institute) for donating mice for the micro-CT studies, Kerrie Jara (Baylor College of Medicine) for editorial assistance, Jacob Couturier (Department of Internal Medicine, UT Health, Houston, TX< USA) for help with the flow cytometry and the Mouse Metabolism Core at Baylor College of Medicine (under the Diabetes Research Center; supported by P30 DK079638) for help with insulin and glucose measurements. The authors also thank the human study participants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Khan, I., Perrard, XY., Brunner, G. et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes 39, 1607–1618 (2015). https://doi.org/10.1038/ijo.2015.104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.104
This article is cited by
-
Heterogeneity of circulating CXCR5-PD-1hiTph cells in patients of type 2 and type 1 diabetes in Chinese population
Acta Diabetologica (2023)
-
The antigen CD300e drives T cell inflammation in adipose tissue and elicits an antibody response predictive of the insulin sensitivity recovery in obese patients
Journal of Inflammation (2022)
-
Adaptive immune cells shape obesity-associated type 2 diabetes mellitus and less prominent comorbidities
Nature Reviews Endocrinology (2022)
-
Effects of 16 months of high intensity resistance training on thigh muscle fat infiltration in elderly men with osteosarcopenia
GeroScience (2021)
-
High extracellular ATP levels released through pannexin-1 channels mediate inflammation and insulin resistance in skeletal muscle fibres of diet-induced obese mice
Diabetologia (2021)