Abstract
Background/Objectives:
Timing of food intake associates with body weight regulation, insulin sensitivity and glucose tolerance. However, the mechanism is unknown. The aim of this study was to investigate the effects of changes in meal timing on energy-expenditure, glucose-tolerance and circadian-related variables.
Subjects/Methods:
Thirty-two women (aged 24±4 years and body mass index 22.9±2.6 kg m−2) completed two randomized, crossover protocols: one protocol (P1) including assessment of resting-energy expenditure (indirect-calorimetry) and glucose tolerance (mixed-meal test) (n=10), the other (P2) including circadian-related measurements based on profiles in salivary cortisol and wrist temperature (Twrist) (n=22). In each protocol, participants were provided with standardized meals (breakfast, lunch and dinner) during the two meal intervention weeks and were studied under two lunch-eating conditions: Early Eating (EE; lunch at 13:00) and Late Eating (LE; lunch 16:30).
Results:
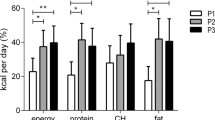
LE, as compared with EE, resulted in decreased pre-meal resting-energy expenditure (P=0.048), a lower pre-meal protein-corrected respiratory quotient (CRQ) and a changed post-meal profile of CRQ (P=0.019). These changes reflected a significantly lower pre-meal utilization of carbohydrates in LE versus EE (P=0.006). LE also increased glucose area under curve above baseline by 46%, demonstrating decreased glucose tolerance (P=0.002). Changes in the daily profile of cortisol and Twrist were also found with LE blunting the cortisol profile, with lower morning and afternoon values, and suppressing the postprandial Twrist peak (P<0.05).
Conclusions:
Eating late is associated with decreased resting-energy expenditure, decreased fasting carbohydrate oxidation, decreased glucose tolerance, blunted daily profile in free cortisol concentrations and decreased thermal effect of food on Twrist. These results may be implicated in the differential effects of meal timing on metabolic health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW . Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009; 17: 2100–2102.
Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA . Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci 2009; 106: 4453–4458.
Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA 2010; 107: 18664–18669.
Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C . Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 2010; 151: 1019–1029.
Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA . Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013; 37: 604–611.
Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M . Entrainment of the circadian clock in the liver by feeding. Science (New York, NY) 2001; 291: 490–493.
Patton DF, Mistlberger RE . Circadian adaptations to meal timing: neuroendocrine mechanisms. Front Neurosci 2013; 7: 185.
Corbalan-Tutau MD, Madrid JA, Ordovas JM, Smith CE, Nicolas F, Garaulet M . Differences in daily rhythms of wrist temperature between obese and normal-weight women: associations with metabolic syndrome features. Chronobiol Int 2011; 28: 425–433.
Bandin C, Martinez-Nicolas A, Ordovas JM, Madrid JA, Garaulet M . Circadian rhythmicity as a predictor of weight-loss effectiveness. Int J Obes (Lond) 2013; 38: 1083–1088.
Jakubowicz D, Barnea M, Wainstein J, Froy O . High caloric intake at breakfast vs dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013; 21: 2504–2512.
Boulos Z, Terman M . Food availability and daily biological rhythms. Neurosci Biobehav Rev 1979; 4: 119–131.
Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, Buijs RM, Escobar C . The suprachiasmatic nucleus participates in food entrainment: a lesion study. Neuroscience 2010; 165: 1115–1126.
Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008; 105: 20970–20975.
Perez-Llamas F, Garaulet M, Torralba C, Zamora S . [Development of a current version of a software application for research and practice in human nutrition (GRUNUMUR 2.0)]. Nutr Hosp 2012; 27: 1576–1582.
Sarabia JA, Rol MA, Mendiola P, Madrid JA . Circadian rhythm of wrist temperature in normal-living subjects A candidate of new index of the circadian system. Physiol Behav 2008; 95: 570–580.
da Rocha EE, Alves VG, da Fonseca RB . Indirect calorimetry: methodology, instruments and clinical application. Curr Opin Clin Nutr Metabol Care 2006; 9: 247–256.
Weir JB . New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109: 1–9.
Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX . The use of areas under curves in diabetes research. Diabetes Care 1995; 18: 245–250.
Ortiz-Tudela E, Martinez-Nicolas A, Campos M, Rol MA, Madrid JA . A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput Biol 2010; 6: e1000996.
Kirschbaum C, Hellhammer DH . Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 1994; 19: 313–333.
Rizzo MR, Mari D, Barbieri M, Ragno E, Grella R, Provenzano R et al. Resting metabolic rate and respiratory quotient in human longevity. J Clin Endocrinol Metabol 2005; 90: 409–413.
Lewis GF, McNally C, Blackman JD, Polonsky KS, Barron WM . Prior feeding alters the response to the 50-g glucose challenge test in pregnancy. The Staub-Traugott effect revisited. Diabetes Care 1993; 16: 1551–1556.
Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS . Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 1991; 88: 934–942.
Leproult R, Holmback U, Van Cauter E . Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014; 63: 1860–1869.
Gonnissen HK, Rutters F, Mazuy C, Martens EA, Adam TC, Westerterp-Plantenga MS . Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr 2012; 96: 689–697.
Chrousos GP, Gold PW . A healthy body in a healthy mind—and vice versa—the damaging power of ‘uncontrollable’ stress. J Clin Endocrinol Metabol 1998; 83: 1842–1845.
Lipiner-Friedman D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M et al. Adrenal function in sepsis: the retrospective Corticus cohort study. Crit Care Med 2007; 35: 1012–1018.
Garcia-Prieto MD, Tebar FJ, Nicolas F, Larque E, Zamora S, Garaulet M . Cortisol secretary pattern and glucocorticoid feedback sensitivity in women from a Mediterranean area: relationship with anthropometric characteristics, dietary intake and plasma fatty acid profile. Clin Endocrinol 2007; 66: 185–191.
Corbalan-Tutau D, Madrid JA, Nicolas F, Garaulet M . Daily profile in two circadian markers ‘melatonin and cortisol’ and associations with metabolic syndrome components. Physiol Behav 2014; 123: 231–235.
Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, Androulakis IP . Entrainment of peripheral clock genes by cortisol. Physiol Genomics 2012; 44: 607–621.
Linkowski P, Onderbergen AV, Kerkhofs M, Bosson D, Mendlewicz J, Cauter EV . Twin study of the 24-h cortisol profile evidence for genetic control of the human circadian clock. Am J Physiol 1993; 264 (2 Pt 1): WI 53190 USA E173–E181.
Corbalán-Tutau MD, Gómez-Abellán P, Madrid JA, Canteras M, Ordovás JM, Garaulet M . Toward a chronobiological characterization of obesity and metabolic syndrome in clinical practice. Clin Nutr 2014. In Press.
Acknowledgements
This work was supported by The Spanish Government of Science and Innovation (Project No. BFU2011-24720 and BFU2010-21945-C02-01) and Seneca Foundation (Project No. 15123/PI/10). Frank A.J.L. Scheer was supported in part by grants NHLBI R01 HL094806 and NIDDK R01 DK099512.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Bandín, C., Scheer, F., Luque, A. et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int J Obes 39, 828–833 (2015). https://doi.org/10.1038/ijo.2014.182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.182
This article is cited by
-
Maternal melatonin levels and temporal dietary intake: results from MY-CARE cohort study
BMC Pregnancy and Childbirth (2023)
-
Isocaloric diet is as effective as the hypocaloric diet in ameliorating symptoms in PCOS patients
International Journal of Diabetes in Developing Countries (2023)
-
Integration of Time-Based Recommendations with Current Pediatric Health Behavior Guidelines: Implications for Obesity Prevention and Treatment in Youth
Current Obesity Reports (2022)
-
Behavioral circadian phenotypes are associated with the risk of elevated body mass index
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2022)
-
Der Genotyp diktiert die Glukosetoleranz bei Spätessern mit
Info Diabetologie (2022)