Abstract
Background:
Bile acids (BAs) are nutrient-responsive hormones that modulate energy balance through cell surface and nuclear receptors. Postprandial plasma BAs have been found to be decreased in obesity.
Objective:
We aimed to determine whether meal-stimulated circulating BA levels are altered by Roux-en-Y gastric bypass (RYGB), an operation that modifies the neurohumoral determinants of food intake and energy expenditure to cause significant and durable weight loss.
Design:
Longitudinal study measuring fasting and postprandial plasma BAs before and after RYGB.
Subjects:
Five obese surgical patients and eight lean controls underwent frequent blood sampling after a standard liquid meal. Obese subjects were also tested at 1, 4 and 40 weeks after RYGB. Primary and secondary circulating BAs, as well as their glycine and taurine conjugates, were measured via reverse-phase high-performance liquid chromatography/mass spectroscopy.
Results:
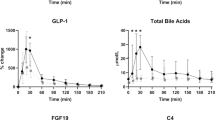
We found that postprandial excursion of conjugated BAs was 52.4% lower in obese than in lean individuals by area-under-the-curve (AUC) analysis (378 vs 793 μmol min l−1, respectively, P<0.05). By 40 weeks after RYGB, the meal-induced rise in conjugated BAs increased by 55.5% to the level of healthy lean controls (378 pre-op vs 850 μmol min l− post-op by AUC analyses, P<0.05). In contrast, postprandial concentrations of unconjugated BAs were similar in lean and obese individuals and were not affected by surgery.
Conclusion:
In light of the growing evidence that BAs have key roles in glucose, lipid and energy homeostasis, the observation that RYGB normalizes the blunted postprandial circulating BA response in obesity suggests that BAs may contribute to the improvement in meal-related physiology seen after RYGB. Further studies are warranted to examine this hypothesis and to determine the degree to which an augmented BA response to nutrient ingestion may mediate the increased incretin response, brown adipose tissue activation and thermic effect of feeding that has been observed after this operation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Spiegelman BM, Flier JS . Obesity and the regulation of energy balance. Cell 2001; 104: 531–543.
Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P . Bile acids as regulatory molecules. J. Lipid Res 2009; 50: 1509–1520.
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B . Role of BAs and BA receptors in metabolic regulation. Physiol Rev 2009; 89: 147–191.
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A et al. Identification of a nuclear receptor for BAs. Science 1999; 284: 1362–1365.
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA et al. BAs: natural ligands for an orphan nuclear receptor. Science 1999; 284: 1365–1368.
Wang H, Chen J, Hollister K, Sowers LC, Forman BM . Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 1999; 3: 543–553.
Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 2002; 298: 714–719.
Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M et al. A G protein-coupled receptor responsive to BAs. J Biol Chem 2003; 278: 9435–9440.
Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 2009; 17: 1671–1677.
Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H . Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009; 58: 1400–1407.
Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012; 153: 3613–3619.
Lundasen T, Galman C, Angelin B, Rudling M . Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med 2006; 260: 530–536.
Wu A, Coulter S, Liddle C, Wong A, Eastham-Anderson J, French DM et al. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. Plos One 2011; 6: e17868.
Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K et al. FGF19 as a postprandial insulin-independent activator of hepatic protein and glycogen synthesis. Science 2011; 6024: 1621–1624.
Katsuma S, Hirasawa A, Tsujimoto G . Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 2005; 329: 386–390.
Thomas C, Gioiella A, Noriega L, Strehle A, Oury J, Rizzo G . TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009; 10: 167–177.
LaRusso NF, Korman MG, Hoffman NE, Hofmann AF . Dynamics of the enterohepatic circulation of bile acids: postprandial serum concentrations of conjugates of cholic acid in health, cholecystectomized patients, and patients with bile acid malabsorption. N Engl J Med 1974; 291: 689–692.
Van Berge-Henegouwen GP, Hofmann AF . Systemic spill-over of bile acids. Euro J Clin Invest 1983; 13: 433–437.
Angelin B, Bjorkhem I, Einarsson K, Ewerth S . Hepatic uptake of bile acids in man: fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest 1982; 70: 724–731.
Greenfield SM, Soloway RD, Carithers RL, Soper K, Silva de Barros SG, Balistreri WF . Evaluation of postprandial serum bile acid response as a test of hepatic function. Dig Dis Sci 1986; 31: 785–791.
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006; 439: 484–489.
Ockenga J, Valentini L, Schuetz T, Wohlgemuth F, Glaesar S, Omar A et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J Clin Endocrinol Metab 2012; 97: 535–542.
Glicksman C, Pournaras DJ, Wright M, Roberts R, Mahon D, Welbourn R et al. Postprandial plasma bile acid responses in normal weight and obese subjects. Ann Clin Biochem 2010; 47: 482–484.
Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes 2009; 33: 786–795.
Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab 2009; 94: 884–891.
Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716.
Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006; 91: 1735–1740.
Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity 2011; 19: 2149–2157.
Stylopoulos N, Hoppin AG, Kaplan LM . Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity 2009; 17: 1839–1847.
Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR et al. Gastric bypass increases energy expenditure in rats. Gastroenterology 2010; 138: 1845–1853.
Faria SL, Faria OP, de Almeida Cardeal M, de Gouvea HR, Buffington C . Diet-induced thermogenesis and respiratory quotient after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2012; 8: 797–802.
Wilms B, Ernst B, Thurnheer M, Schultes B . Increased thermic effect of food after gastric bypass surgery. Obes Rev 2012; 13 (Suppl 2): 125.
Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr 2003; 78: 22–30.
Thivel D, Brakonieki K, Duche P, Beatrice M, B Yves, Laferrere B . Surgical weight loss: impact on energy expenditure. Obes Surg 2013; 23: 255–266.
Rosenbaum M, Hirsch J, Gallagher DA, Leibel R . Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 2008; 88: 906–912.
Stylopoulos N, Zhang XB, Brownell AL, Kaplan LM . Roux-en-Y gastric bypass activates brown adipose tissue and increases energy expenditure in obese mice. Gastroenterology 2010; 138 (Suppl 1): S754 (abstract W1854).
Nestoridi E, Kvas S, Kucharczyk J, Stylopoulos N . Resting energy expenditure and energetic cost of feeding are augmented after Roux en Y gastric bypass in obese mice. Endocrinology 2012; 153: 2234–2244.
Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected brown adipose tissue in humans. J Clin Endocrinol Metab 2011; 96: 192–199.
Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 2010; 59: 1789–1793.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts J, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
Thompson FE, Byers T . Dietary assessment resource manual. J Nutr 1994; 124: 2245S–2317S.
Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Theriault G . A method to assess energy expenditure in children and adults. Am J Clin Nutr 1983; 37: 461–467.
Bobeldijk I, Hekman M, de Vries-van der Weij J, Coulier L, Ramaker R, Kleemann R et al. Quantitative profiling of bile acids in biofluids and tissues based on accurate mass high resolution LC-FT-MS: compound class targeting in a metabolomics workflow. J Chromatogr B Analyt Technol Biomed Life Sc 2008; 871: 306–313.
Tai MM . A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diab Care 1994; 17: 152–154.
Hatoum IJ, Greenawalt DM, Cotsapas C, Reitman ML, Daly MJ, Kaplan LM . Heritability of the weight loss response to gastric bypass surgery. J Clin Endocrinol Metab 2011; 96: E1630–E1633.
Sato H, Macchairulo A, Thomas C, Gioiello A, Une M, Hofmann AF et al. Novel potent and selective BA derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem 2008; 51: 1831–1841.
Kohli R, Kirby M, Setchell KDR, Jha P, Klustaitis K, Woollett LA et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol 2010; 299: 652–660.
Patriti A, Facchiano E, Annetti C, Aisa M, Galli F, Fanelli C et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg 2005; 15: 1258–1264.
Boza C, Munoz R, Yung E, Milone L, Gagner M . Sleeve gastrectomy with ileal transposition induces a significant weight loss and diabetes improvement without exclusion of the proximal intestine. J Gastrointest Surg 2011; 15: 928–934.
Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J et al. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol 2006; 290: 923–932.
Vanwijngaerden Y, Wauters J, Langouche L, Perre SV, Liddle C, Coulter S et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology 2011; 54: 1741–1752.
De La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge C, Raybould HE . Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 2010; 299: 440–448.
Furet J, Kong L, Tap J, Poitou C, Basdevant A, Bouillot J et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010; 59: 3049–3057.
Liou AP, Paziuk M, Luevano JM, Machineni S, Turnbaugh PJ, Kaplan LM . Conserved shifts in the gut microbiota caused by Roux-en-Y gastric bypass contribute to reduced host weight and adiposity. Sci Transl Med 2013; 5, 178ra41 1–11.
Novais PF, Rasera I Jr, Leite CV, Marin FA, de Oliveira MR . Food intake in women two years or more after bariatric surgery meets adequate intake requirements. Nutr Res 2012; 32: 335–341.
Acknowledgements
This study was supported by grants DK083230, KL2 RR025757, DK08866, DK090956 and UL1 RR025758 from the National Institutes of Health and a research grant from Ethicon Endo-Surgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was presented in part at the annual meeting of the American Gastroenterological Association in May 2010.
Rights and permissions
About this article
Cite this article
Ahmad, N., Pfalzer, A. & Kaplan, L. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes 37, 1553–1559 (2013). https://doi.org/10.1038/ijo.2013.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.38
Keywords
This article is cited by
-
Poly-Agonist Pharmacotherapies for Metabolic Diseases: Hopes and New Challenges
Drugs (2024)
-
The role of the gut microbiota in health and cardiovascular diseases
Molecular Biomedicine (2022)
-
Intestinal plasticity and metabolism as regulators of organismal energy homeostasis
Nature Metabolism (2022)
-
Metabolomic signatures after bariatric surgery – a systematic review
Reviews in Endocrine and Metabolic Disorders (2022)
-
Reversal of NAFLD After VSG Is Independent of Weight-Loss but RYGB Offers More Efficacy When Maintained on a High-Fat Diet
Obesity Surgery (2022)