Abstract
Background:
It has been suggested that the metabolic benefits of physical exercise could be mediated by myokines. We examined here the effect of exercise training on skeletal muscle expression of a panel of myokines in humans. Pathways regulating myokine expression were investigated in human myotubes.
Methods:
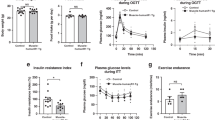
Eleven obese non-diabetic male subjects were enrolled in an 8-week endurance training program. Insulin sensitivity was assessed by an oral glucose tolerance test. Subcutaneous adipose tissue and Vastus lateralis muscle biopsy samples were collected before and after training. RNAs were prepared from adipose tissue and skeletal muscle. Primary culture of myoblasts was established.
Results:
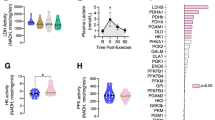
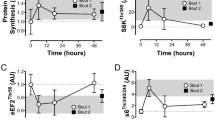
As expected, exercise training improved aerobic capacity and decreased fat mass. No significant change in interleukin 6, fibroblast growth factor 21, myostatin (MSTN) or irisin mRNA level was found in muscle after training. A twofold increase in apelin mRNA level was found in muscle but not in adipose tissue. No change in circulating myokine and adipokine plasma levels was observed in the resting state in response to training. Interestingly, apelin was significantly expressed and secreted in primary human myotubes. Apelin gene expression was upregulated by cyclic AMP and calcium, unlike the other myokines investigated. Importantly, changes in muscle apelin mRNA levels were positively related to whole-body insulin sensitivity improvement.
Conclusion:
Collectively, our data show that exercise training upregulates muscle apelin expression in obese subjects. Apelin expression is induced by exercise signaling pathways and secreted in vitro in human primary myotubes, and may behave as a novel exercise-regulated myokine with autocrine/paracrine action.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Blair SN, Church TS . The fitness, obesity, and health equation: is physical activity the common denominator? JAMA 2004; 292: 1232–1234.
Egan B, Zierath JR . Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013; 17: 162–184.
Holloszy JO . Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol 2008; 59 (Suppl 7): 5–18.
Hood DA . Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab 2009; 34: 465–472.
Rockl KS, Witczak CA, Goodyear LJ . Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life 2008; 60: 145–153.
Pedersen BK, Febbraio MA . Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8: 457–465.
Febbraio MA . Signaling pathways for IL-6 within skeletal muscle. Exerc Immunol Rev 2003; 9: 34–39.
Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006; 55: 2688–2697.
McPherron AC, Lawler AM, Lee SJ . Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997; 387: 83–90.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468.
Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K . FGF21 is an Akt-regulated myokine. FEBS Lett 2008; 582: 3805–3810.
Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F . Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab 2010; 11: 206–212.
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–1635.
Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D . Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009; 32: 1542–1546.
Cuevas-Ramos D, Almeda-Valdes P, Meza-Arana CE, Brito-Cordova G, Gomez-Perez FJ, Mehta R et al. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 2012; 7: e38022.
Hittel DS, Axelson M, Sarna N, Shearer J, Huffman KM, Kraus WE . Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc 2010; 42: 2023–2029.
Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012; 61: 1725–1738.
Yang SJ, Hong HC, Choi HY, Yoo HJ, Cho GJ, Hwang TG et al. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin Endocrinol (Oxf) 2011; 75: 464–469.
de Glisezinski I, Moro C, Pillard F, Marion-Latard F, Harant I, Meste M et al. Aerobic training improves exercise-induced lipolysis in SCAT and lipid utilization in overweight men. Am J Physiol Endocrinol Metab 2003; 285: E984–E990.
Marquez-Quinones A, Mutch DM, Debard C, Wang P, Combes M, Roussel B et al. Adipose tissue transcriptome reflects variations between subjects with continued weight loss and subjects regaining weight 6 mo after caloric restriction independent of energy intake. Am J Clin Nutr 2010; 92: 975–984.
Moro C, Galgani JE, Luu L, Pasarica M, Mairal A, Bajpeyi S et al. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J Clin Endocrinol Metab 2009; 94: 3440–3447.
Taniguchi A, Fukushima M, Nagasaka S, Matsumoto K, Tokuyama K, Doi K et al. Insulin sensitivity indexes from a single sample in nonobese Japanese type 2 diabetic patients: comparison with minimal model analysis. Diabetes Care 2002; 25: 626–640.
Pillard F, Van Wymelbeke V, Garrigue E, Moro C, Crampes F, Guilland JC et al. Lipid oxidation in overweight men after exercise and food intake. Metabolism 2010; 59: 267–274.
Larrouy D, Barbe P, Valle C, Dejean S, Pelloux V, Thalamas C et al. Gene expression profiling of human skeletal muscle in response to stabilized weight loss. Am J Clin Nutr 2008; 88: 125–132.
Viguerie N, Picard F, Hul G, Roussel B, Barbe P, Iacovoni JS et al. Multiple effects of a short-term dexamethasone treatment in human skeletal muscle and adipose tissue. Physiol Genomics 2012; 44: 141–151.
Badin PM, Louche K, Mairal A, Liebisch G, Schmitz G, Rustan AC et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 2011; 60: 1734–1742.
Castan-Laurell I, Dray C, Knauf C, Kunduzova O, Valet P . Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol Metab 2012; 23: 234–241.
Konopka AR, Douglass MD, Kaminsky LA, Jemiolo B, Trappe TA, Trappe S et al. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci 2010; 65: 1201–1207.
Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 2013; 98: E769–E778.
Timmons JA, Baar K, Davidsen PK, Atherton PJ . Is irisin a human exercise gene? Nature 2012; 488: E9–E10 discussion E10–11.
Lambert CP, Wright NR, Finck BN, Villareal DT . Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol 2008; 105: 473–478.
Krist J, Wieder K, Kloting N, Oberbach A, Kralisch S, Wiesner T et al. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obes Facts 2013; 6: 57–69.
Kadoglou NP, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS . The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with Type 2 diabetes. Diabet Med 2013; 30: e41–e50.
Kadoglou NP, Vrabas IS, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monit 2012; 18: CR290–CR295.
Yamamoto T, Habata Y, Matsumoto Y, Yasuhara Y, Hashimoto T, Hamajyo H et al. Apelin-transgenic mice exhibit a resistance against diet-induced obesity by increasing vascular mass and mitochondrial biogenesis in skeletal muscle. Biochim Biophys Acta 2011; 1810: 853–862.
Akerstrom T, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK . Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol 2005; 563: 507–516.
Kotnik P, Fischer-Posovszky P, Wabitsch M . Eur J Endocrinol 2011; 165: 703–711.
Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buleon M et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab 2008; 8: 437–445.
Sparks LM, Moro C, Ukropcova B, Bajpeyi S, Civitarese AE, Hulver MW et al. Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS One 2011; 6: e21068.
Buford TW, Cooke MB, Willoughby DS . Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur J Appl Physiol 2009; 107: 463–471.
Barnes G, Japp AG, Newby DE . Translational promise of the apelin-APJ system. Heart 2010; 96: 1011–1016.
Mesmin C, Dubois M, Becher F, Fenaille F, Ezan E . Liquid chromatography/tandem mass spectrometry assay for the absolute quantification of the expected circulating apelin peptides in human plasma. Rapid Commun Mass Spectrom 2010; 24: 2875–2884.
Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab 2010; 298: E59–E67.
Acknowledgements
We are very grateful to the staff of Toulouse Clinical Investigation Centre and to the study participants. This study was supported by grants from the National Research Agency ANR-12-JSV1-0010-01 (CM) and ANR-09-GENO-0018-01 (DL), European Federation for the Study of Diabetes/Novo Nordisk and Société Francophone du Diabète (CM), Inserm DHOS Recherche Translationnelle and AOL Hôpitaux de Toulouse (DL), Fondation pour la Recherche Médicale (DL), Inserm DHOS Recherche Translationnelle 2009 (CT, DL), AOL 08 163 02 Hôpitaux de Toulouse (CT, DL) and Glaxo Smith Kline (DL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Besse-Patin, A., Montastier, E., Vinel, C. et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes 38, 707–713 (2014). https://doi.org/10.1038/ijo.2013.158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.158
Keywords
This article is cited by
-
Gpcpd1–GPC metabolic pathway is dysfunctional in aging and its deficiency severely perturbs glucose metabolism
Nature Aging (2024)
-
Epidemiological, mechanistic, and practical bases for assessment of cardiorespiratory fitness and muscle status in adults in healthcare settings
European Journal of Applied Physiology (2023)
-
Myokine, a key cytokine for physical exercise to alleviate sarcopenic obesity
Molecular Biology Reports (2023)
-
Irisin: circulating levels in serum and its relation to gonadal axis
Endocrine (2022)
-
The effect of 12 weeks of training in water on serum levels of SIRT1 and FGF-21, glycemic index, and lipid profile in patients with type 2 diabetes
International Journal of Diabetes in Developing Countries (2022)