Abstract
Background:
Adipose tissue is critical for systemic metabolic health. Identifying key factors regulating adipose tissue function is a research priority. The NR4A subfamily of nuclear receptors (NRs) (NR4A1/NUR77, NR4A2/NURR1 and NR4A3/NOR1) has emerged as important proteins in different disease states and in the regulation of metabolic tissues, particularly in liver and muscle. However, the expression of the NR4A members in human adipose tissue has not previously been described, and their target genes are largely unknown.
Objective:
To determine whether the NR4As are differentially expressed in human adipose tissue in obesity, and identify potential NR4A target genes.
Design:
Prospective analysis of s.c. adipose tissue before and 1 year after fat loss, and during in vitro differentiation of primary human preadipocytes. Case-control comparison of omental (OM) adipose tissue.
Subjects:
A total of 13 extremely obese patients undergoing biliopancreatic diversion with duodenal switch for fat loss, 12 extremely obese patients undergoing laparoscopic sleeve gastrectomy and 37 lean individuals undergoing hernia repair or laparotomy were included in the study. Measurements were done by quantitative PCR gene expression analysis of the NR4A members and in silico promoter analysis based on microarray data.
Results:
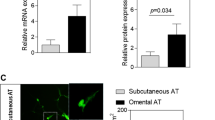
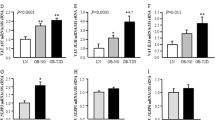
There was a strong upregulation of the NR4As in extreme obesity and normalization after fat loss. The NR4As were expressed at the highest level in stromal–vascular fraction compared with adipocytes, but were downregulated in both fractions after fat loss. Their expression levels were also significantly higher in OM compared with s.c. adipocytes in obesity. The NR4As were downregulated during differentiation of primary human preadipocytes. Moreover, the NR4As were strongly induced within 30 min of tissue incubation. Finally, promoter analysis revealed potential NR4A target genes involved in stress response, immune response, development and other functions. Our data show altered adipose tissue expression of the NR4As in obesity, suggesting that these stress responsive nuclear receptors may modulate pathogenic potential in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Iozzo P . Viewpoints on the way to the consensus session: where does insulin resistance start? The adipose tissue. Diabetes Care 2009; 32 (Suppl 2): S168–S173.
Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008; 6: 343–368.
Hotamisligil GS . Inflammation and metabolic disorders. Nature 2006; 444: 860–867.
Gregor MF, Hotamisligil GS . Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29: 415–445.
McKenna NJ, O’Malley BW . SnapShot: nuclear receptors I. Cell 2010; 142: 822–822.e1.
McKenna NJ, O’Malley BW . SnapShot: nuclear receptors II. Cell 2010; 142: 986.e1.
Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ . Nuclear receptors and lipid physiology: opening the X-files. Science 2001; 294: 1866–1870.
Sonoda J, Pei L, Evans RM . Nuclear receptors: decoding metabolic disease. FEBS Letters 2008; 582: 2–9.
Lonard DM, Lanz RB, O’Malley BW . Nuclear receptor coregulators and human disease. Endocr Rev 2007; 28: 575–587.
O’Malley BW, McKenna NJ . Coactivators and corepressors: what's in a name? Mol Endocrinol 2008; 22: 2213–2214.
Kim MS, Sweeney TR, Shigenaga JK, Chui LG, Moser A, Grunfeld C et al. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism 2007; 56: 267–279.
Fang C, Yoon S, Tindberg N, Jarvelainen HA, Lindros KO, Ingelman-Sundberg M . Hepatic expression of multiple acute phase proteins and down-regulation of nuclear receptors after acute endotoxin exposure. Biochem Pharmacol 2004; 67: 1389–1397.
Maruyama K, Tsukada T, Ohkura N, Bandoh S, Hosono T, Yamaguchi K . The NGFI-B subfamily of the nuclear receptor superfamily (review). Int J Oncol 1998; 12: 1237–1243.
Pearen MA, Muscat GEO . Minireview: nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 2010; 24: 1891–1903.
Maxwell MA, Muscat GE . The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal 2006; 4: e002.
van Tiel CM, de Vries CJM . NR4All in the vessel wall. J Steroid Biochem Mol Biol 2011; e-pub ahead of print 26 January 2011.
Zhao Y, Bruemmer D . NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol 2010; 30: 1535–1541.
Pols TW, Bonta PI, de Vries CJ . NR4A nuclear orphan receptors: protective in vascular disease? Curr Opin Lipidol 2007; 18: 515–520.
McMorrow JP, Murphy EP . Inflammation: a role for NR4A orphan nuclear receptors? Biochem Soc Trans 2011; 39: 688–693.
Maira M, Martens C, Batsche E, Gauthier Y, Drouin J . Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol 2003; 23: 763–776.
Perlmann T, Jansson L . A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev 1995; 9: 769–782.
Dankel SN, Fadnes DJ, Stavrum AK, Stansberg C, Holdhus R, Hoang T et al. Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss. PLoS One 2010; 5: e11033.
van Harmelen V, Skurk T, Hauner H . Primary culture and differentiation of human adipocyte precursor cells. Methods Mol Med 2005; 107: 125–135.
Fu Y, Luo L, Luo N, Zhu X, Garvey WT . NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistance. J Biol Chem 2007; 282: 31525–31533.
Au WS, Payne VA, O’Rahilly S, Rochford JJ . The NR4A family of orphan nuclear receptors are not required for adipogenesis. Int J Obes (Lond) 2008; 32: 388–392.
Chao LC, Bensinger SJ, Villanueva CJ, Wroblewski K, Tontonoz P . Inhibition of adipocyte differentiation by Nur77, Nurr1, and Nor1. Mol Endocrinol 2008; 22: 2596–2608.
Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 2007; 92: 2240–2247.
Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M et al. Insulin sensitive obesity. Am J Physiol Endocrinol Metab 2010; 299: E506–E515.
Yamashita S, Tanaka Y, Tsutsumi S, Aburatani H, Minato N, Ihara S . Analysis of mechanism for human gammadelta T cell recognition of nonpeptide antigens. Biochem Biophys Res Commun 2005; 334: 349–360.
Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, Muscat GE . Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem 2005; 280: 12573–12584.
Pei L, Castrillo A, Tontonoz P . Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol Endocrinol 2006; 20: 786–794.
Dalmas E, Clement K, Guerre-Millo M . Defining macrophage phenotype and function in adipose tissue. Trends Immunol 2011; 32: 307–314.
Kim BY, Kim H, Cho EJ, Youn HD . Nur77 upregulates HIF-alpha by inhibiting pVHL-mediated degradation. Exp Mol Med 2008; 40: 71–83.
Wood IS, de Heredia FP, Wang B, Trayhurn P . Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc 2009; 68: 370–377.
Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 2009; 29: 4467–4483.
Trayhurn P, Wang B, Wood IS . Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 2008; 100: 227–235.
Oita RC, Mazzatti DJ, Lim FL, Powell JR, Merry BJ . Whole-genome microarray analysis identifies up-regulation of Nr4a nuclear receptors in muscle and liver from diet-restricted rats. Mech Ageing Dev 2009; 130: 240–247.
Pols TW, Ottenhoff R, Vos M, Levels JH, Quax PH, Meijers JC et al. Nur77 modulates hepatic lipid metabolism through suppression of SREBP1c activity. Biochem Biophys Res Commun 2008; 366: 910–916.
Kolehmainen M, Vidal H, Alhava E, Uusitupa MI . Sterol regulatory element binding protein 1c (SREBP-1c) expression in human obesity. Obes Res 2001; 9: 706–712.
Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P . NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 2006; 12: 1048–1055.
Kumar N, Liu D, Wang H, Robidoux J, Collins S . Orphan nuclear receptor NOR-1 enhances 3′,5′-cyclic adenosine 5′-monophosphate-dependent uncoupling protein-1 gene transcription. Mol Endocrinol 2008; 22: 1057–1064.
Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM et al. A nuclear receptor Atlas: 3T3-L1 adipogenesis. Mol Endocrinol 2005; 19: 2437–2450.
Martinez-Gonzalez J, Badimon L . The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res 2005; 65: 609–618.
Tanabe Y, Koga M, Saito M, Matsunaga Y, Nakayama K . Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPARgamma2. J Cell Sci 2004; 117 (Part 16): 3605–3614.
Acknowledgements
We thank the participants who gave blood samples and tissue biopsies. We thank Tone Saeterdal Myhra, Carol Cook and Anita Ivarsflaten for expert technical assistance. We thank the staff at Voss Hospital, Norway for assistance with biopsy collection, in particular Borghild Strønen, Tor Nedrebø, Maria Decap, Beate Tveit, Olav Lødemel, Åse Hommedal and Hege Sunde Jordalen. We thank the staff at Haraldsplass Deaconess Hospital, Bergen, Norway, particularly Drs. Inge Glambek, Barbara Auras Jaatun and Torodd Bruland. This project was funded by Samarbeidsorganet Helse Vest, Meltzerfondet and Programstyret for ernaering at the University of Bergen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Veum, V., Dankel, S., Gjerde, J. et al. The nuclear receptors NUR77, NURR1 and NOR1 in obesity and during fat loss. Int J Obes 36, 1195–1202 (2012). https://doi.org/10.1038/ijo.2011.240
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.240
Keywords
This article is cited by
-
Spatial and single-cell profiling of the metabolome, transcriptome and epigenome of the aging mouse liver
Nature Aging (2023)
-
The role of NURR1 in metabolic abnormalities of Parkinson’s disease
Molecular Neurodegeneration (2022)
-
COL6A3 expression in adipose tissue cells is associated with levels of the homeobox transcription factor PRRX1
Scientific Reports (2020)
-
Paternal chronic colitis causes epigenetic inheritance of susceptibility to colitis
Scientific Reports (2016)
-
Grape seed procyanidin supplementation to rats fed a high-fat diet during pregnancy and lactation increases the body fat content and modulates the inflammatory response and the adipose tissue metabolism of the male offspring in youth
International Journal of Obesity (2015)