Abstract
Aims:
The pathogenesis of obesity remains incompletely understood and the exploration of the role of novel proteins in obesity may provide important insights into its causes and treatments. Here, we report a previously unidentified role for synphilin-1 in the control of food intake and body weight. Synphilin-1, a cytoplasmic protein, was initially identified as an interaction partner of alpha-synuclein, and has implications in Parkinson's disease pathogenesis related to protein aggregation.
Subjects and methods:
To study the in vivo role of synphilin-1, we characterized a human synphilin-1 transgenic mouse (SP1) by assessing synphilin-1 expression, plasma parameters, food intake and spontaneous activity to determine the major behavioral changes and their consequences in the development of the obesity phenotype.
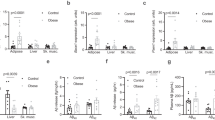
Results:
Expression of human synphilin-1 in brain neurons in SP1 mice resulted in increased food intake, body weight and body fat. SP1 mice also displayed hyperinsulinemia, hyperleptinemia and impaired glucose tolerance. Pair-feeding SP1 mice to amounts consumed by non-transgenic mice prevented the increased body weight, adiposity, hyperinsulinemia and hyperleptinemia demonstrating that these were all the consequences of increased food intake. Transgenic expression of synphilin-1 was enriched in hypothalamic nuclei involved in feeding control, and fasting-induced elevated endogenous synphilin-1 levels at these sites, suggesting that synphilin-1 is an important player in the hypothalamic energy balance regulatory system.
Conclusion:
These studies identify a novel function of synphilin-1 in controlling food intake and body weight, and may provide a unique obesity model for future studies of obesity pathogenesis and therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ et al. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet 1999; 22: 110–114.
Ribeiro CS, Carneiro K, Ross CA, Menezes JR, Engelender S . Synphilin-1 is developmentally localized to synaptic terminals, and its association with synaptic vesicles is modulated by alpha-synuclein. J Biol Chem 2002; 277: 23927–23933.
Nagano Y, Yamashita H, Takahashi T, Kishida S, Nakamura T, Iseki E et al. Siah-1 facilitates ubiquitination and degradation of synphilin-1. J Biol Chem 2003; 278: 51504–51514.
Wakabayashi K, Engelender S, Yoshimoto M, Tsuji S, Ross CA, Takahashi H . Synphilin-1 is present in Lewy bodies in Parkinson's disease. Ann Neurol 2000; 47: 521–523.
Bandopadhyay R, Kingsbury AE, Muqit MM, Harvey K, Reid AR, Kilford L et al. Synphilin-1 and parkin show overlapping expression patterns in human brain and form aggresomes in response to proteasomal inhibition. Neurobiol Dis 2005; 20: 401–411.
Smith WW, Margolis RL, Li X, Troncoso JC, Lee MK, Dawson VL et al. Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J Neurosci 2005; 25: 5544–5552.
Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J et al. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 2001; 7: 1144–1150.
Szargel R, Rott R, Engelender S . Synphilin-1 isoforms in Parkinson's disease: regulation by phosphorylation and ubiquitylation. Cell Mol Life Sci 2008; 65: 80–88.
O’Farrell C, Murphy DD, Petrucelli L, Singleton AB, Hussey J, Farrer M et al. Transfected synphilin-1 forms cytoplasmic inclusions in HEK293 cells. Brain Res Mol Brain Res 2001; 97: 94–102.
Lee G, Tanaka M, Park K, Lee SS, Kim YM, Junn E et al. Casein kinase II-mediated phosphorylation regulates alpha-synuclein/synphilin-1 interaction and inclusion body formation. J Biol Chem 2004; 279: 6834–6839.
Avraham E, Szargel R, Eyal A, Rott R, Engelender S . Glycogen synthase kinase 3beta modulates synphilin-1 ubiquitylation and cellular inclusion formation by SIAH: implications for proteasomal function and Lewy body formation. J Biol Chem 2005; 280: 42877–42886.
Marx FP, Soehn AS, Berg D, Melle C, Schiesling C, Lang M et al. The proteasomal subunit S6 ATPase is a novel synphilin-1 interacting protein--implications for Parkinson's disease. FASEB J 2007; 21: 1759–1767.
varez-Castelao B, Castano JG . Synphilin-1 inhibits alpha-synuclein degradation by the proteasome. Cell Mol Life Sci 2010; 68: 2643–2654.
Li X, Liu Z, Tamashiro K, Shi B, Rudnicki DD, Ross CA et al. Synphilin-1 exhibits trophic and protective effects against Rotenone toxicity. Neuroscience 2010; 165: 455–462.
Giaime E, Sunyach C, Herrant M, Grosso S, Auberger P, McLean PJ et al. Caspase-3-derived C-terminal product of synphilin-1 displays antiapoptotic function via modulation of the p53-dependent cell death pathway. J Biol Chem 2006; 281: 11515–11522.
Smith WW, Liu Z, Liang Y, Masuda N, Swing DA, Jenkins NA et al. Synphilin-1 attenuates neuronal degeneration in the A53T {alpha}-synuclein transgenic mouse model. Hum Mol Genet 2010; 19: 2087–2098.
Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS et al. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala- 53 --> Thr mutation causes neurodegenerative disease with alpha- synuclein aggregation in transgenic mice. Proc Natl Acad Sci USA 2002; 99: 8968–8973.
Pletnikov MV, Rubin SA, Schwartz GJ, Moran TH, Sobotka TJ, Carbone KM . Persistent neonatal Borna disease virus (BDV) infection of the brain causes chronic emotional abnormalities in adult rats. Physiol Behav 1999; 66: 823–831.
Tamashiro KL, Wakayama T, Akutsu H, Yamazaki Y, Lachey JL, Wortman MD et al. Cloned mice have an obese phenotype not transmitted to their offspring. Nat Med 2002; 8: 262–267.
Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S et al. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci USA 2007; 104: 17358–17363.
Tremblay A, Therrien F . Physical activity and body functionality: implications for obesity prevention and treatment. Can J Physiol Pharmacol 2006; 84: 149–156.
Vargas RH, Ornelas LF, Gonzalez IL, Escovar JR, Zurita M, Reynaud E . Synphilin suppresses alpha-synuclein neurotoxicity in a Parkinson's disease Drosophila model. Genesis 2011; 49: 392–402.
Jin HG, Yamashita H, Nakamura T, Fukuba H, Takahashi T, Hiji M et al. Synphilin-1 transgenic mice exhibit mild motor impairments. Neurosci Lett 2008; 445: 12–17.
Krenz A, Falkenburger BH, Gerhardt E, Drinkut A, Schulz JB . Aggregate formation and toxicity by wild-type and R621C synphilin-1 in the nigrostriatal system of mice using adenoviral vectors. J Neurochem 2009; 108: 139–146.
Nuber S, Franck T, Wolburg H, Schumann U, Casadei N, Fischer K et al. Transgenic overexpression of the alpha-synuclein interacting protein synphilin-1 leads to behavioral and neuropathological alterations in mice. Neurogenetics 2010; 11: 107–120.
Ladenheim EE, Behles RR, Bi S, Moran TH . Gastrin-releasing peptide messenger ribonucleic acid expression in the hypothalamic paraventricular nucleus is altered by melanocortin receptor stimulation and food deprivation. Endocrinology 2009; 150: 672–678.
Moran TH . Gut peptide signaling in the controls of food intake. Obesity (Silver Spring) 2006; 14 (Suppl 5): 250S–253S.
Acknowledgements
We thank Yi Yu, Jayson Hyun, Megan Smith and Guangjing Zhu for technical support. We thank Dr T Dawson for read the manuscript. This work was supported by National Institutes of Health, Grants: DK083410 to WWS, NS38377 to CAS, DK19302 and DK068054, and the Paul R. McHugh Chair for Motivated Behavior to THM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, X., Tamashiro, K., Liu, Z. et al. A novel obesity model: synphilin-1-induced hyperphagia and obesity in mice. Int J Obes 36, 1215–1221 (2012). https://doi.org/10.1038/ijo.2011.235
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.235
Keywords
This article is cited by
-
Synphilin-1 alters metabolic homeostasis in a novel Drosophila obesity model
International Journal of Obesity (2012)
-
New role for synphilin-1 in food intake and obesity
Nature Reviews Endocrinology (2012)