Abstract
Objective:
Recent observational studies have reported that body fat distribution might be differentially associated with subclinical atherosclerosis. We previously reported that visceral fat area (VFA) ⩾80 cm2 is the optimal cutoff for identifying abdominal obesity in Chinese subjects. We examined whether VFA ⩾80 cm2 reflects the association between abdominal obesity and subclinical atherosclerosis, and if determination of the visceral fat quantity is useful for assessing subclinical atherosclerosis in asymptomatic individuals.
Methods and results:
Participants (N=1005, men 515, women 490, 34–66 years) free of cardiovascular disease underwent magnetic resonance imaging and carotid ultrasound assessment to quantify VFA and carotid intima–media thickness (C-IMT). Overweight/obese subjects (body mass index (BMI) ⩾25.0 kg m−2) had a higher C-IMT than lean subjects (BMI <25.0 kg m−2) (P<0.01). Subjects with VFA ⩾80 cm2 had significantly higher C-IMT than those without abdominal obesity regardless of BMI (P<0.01). By multivariate regression analysis adjusted for anthropometric measurements and cardiovascular risk factors, waist circumference but not BMI was independently correlated with C-IMT in men (P<0.001). Similar findings were observed with an accurate obesity indices adjusted model, which showed that VFA was an independent risk factor for increased C-IMT in men but not in women.
Conclusions:
VFA ⩾80 cm2 effectively identified carotid atherosclerosis for both lean and obese individuals in middle-aged Chinese men.
Similar content being viewed by others
Introduction
Obesity is a global epidemic and is strongly associated with metabolic disorders and cardiovascular disease (CVD).1 The relationship between obesity and CVD depends not only on the amount of total body fat but also on its distribution.2, 3, 4 In recent years, increasing evidence has shown that, compared with total body fat, visceral fat accumulation is more important for the development of insulin resistance, metabolic syndrome (Mets), type 2 diabetes and CVD.5, 6, 7, 8 Abdominal obesity is considered a fundamental pathology for Mets development, which is associated with increased risk of cardiovascular morbidity and mortality.9, 10
Most studies use waist circumference or waist-to-hip ratio to define abdominal obesity.2, 3, 4, 7, 8 However, measurement of these circumferences cannot distinguish between visceral and subcutaneous adipose tissue. The standard methods for quantifying visceral fat amount recommended by the International Diabetes Federation (IDF) are magnetic resonance imaging (MRI) and computed tomography.11 We previously reported that for a Chinese population, visceral fat area (VFA) ⩾80 cm2 is optimal for detecting two or more metabolic abnormalities. These include hyperglycemia, hypertension and dyslipidemia, using the IDF definition or the 2004 Chinese Diabetes Society definition.12 Whether VFA ⩾80 cm2 reflects an association between abdominal obesity and subclinical atherosclerosis is still unknown. Moreover, to our knowledge, no study has focused on the association between visceral fat amount and the extent of subclinical atherosclerosis in various body mass index (BMI) categories in a Chinese population.
Quantitative assessment of carotid intima–media thickness (C-IMT) is accepted as an indicator of preclinical atherosclerosis and may be used as a marker for cardiovascular mobidity and mortality.13, 14 Therefore, the aims of this study were to: (1) determine if visceral fat accumulation was a stronger risk factor of subclinical atherosclerosis than general obesity in a Chinese population; (2) evaluate whether VFA ⩾80 cm2 was the optimal value to reflect the association between abdominal obesity and subclinical atherosclerosis in both lean and overweight/obese subjects and (3) investigate if visceral fat quantity is useful for assessing subclinical atherosclerosis in asymptomatic individuals.
Subjects and methods
Subjects
A total of 1217 subjects, aged 30 to 70 years, were recruited from December 2009 to June 2010 in Baoshan community, Shanghai, China. The exclusion criteria were: (1) current treatment with systemic corticosteroids; (2) cirrhosis with ascites; (3) known hyperthyroidism or hypothyroidism; (4) presence of cancer; (5) severe disability and psychiatric disturbance; (6) pregnancy and (7) known history of CVD. The excluded were 182 with no MRI data, 3 with no carotid artery scans, 16 with incomplete anthropometric indices and 11 with incomplete lab data or C-reactive protein (CRP) ⩾10 mg l−1. We finally analyzed data from 1005 subjects (men 515, women 490) aged 34–66 years in this study.
All participants were invited to complete a questionnaire about present and past illness and medical therapy. The study was approved by the Ethics Committee of Shanghai Jiaotong University affiliated Sixth People's Hospital. Written informed consent was obtained from all the participants.
Clinical and laboratory assessments of risk factors
A physical examination, including measurement of height, weight, waist circumference (W) and blood pressure (BP), was performed for each participant. BMI was calculated as weight in kilograms divided by the square of height in meters. W was measured at the horizontal plane between the inferior costal margin and the iliac crest on mid-axillary line. Body fat percentage (%fat) was estimated by the TBF-410 Tanita Body Composition Analyzer (Tanita, Tokyo, Japan). BP was the average of three time measurements using a sphygmomanometer at an interval of 3 min.
After a 10-h overnight fast, blood samples were collected to measure plasma glucose level and lipid profile. Subjects without a validated history of diabetes underwent a 75-g oral glucose tolerance test. The 100-g carbohydrate (steamed bread meal) test was performed in diabetic patients.15 Fasting plasma glucose (FPG) and 2-h post-OGTT plasma glucose (2hPG) were assayed by the glucose oxidase method. Serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c) were measured by enzymatic procedures using an autoanalyzer (Hitachi 7600-020, automatic analyzer, Tokyo, Japan). Glycated hemoglobin A1c (HbA1c) was determined by high-pressure liquid chromatography (Bio-Rad Inc., Hercules, CA, USA). The serum concentration of CRP was measured by particle-enhanced immunonephelometry using CardioPhase hs-CRP reagent (Siemens Healthcare Diagnostic Inc., Newark, NJ, USA). The detection limit of the assay was 0.175 mg l−1. The intra-assay coefficients of variation for hs-CRP levels were 2.3%, 4.6%, 2.6% and 2.1% at 0.69, 5.95, 9.23 and 179 mg l−1, respectively; inter-assay coefficients of variation were 2.3%, 4.0%, 1.3% and 1.1% at 0.69, 5.95, 9.23 and 179 mg l−1. Serum insulin concentration was measured by radioimmunoassay (Linco Research, St Charles, MO, USA). Insulin sensitivity was estimated by homeostasis model assessment-insulin resistance (HOMA-IR) based on fasting glucose and insulin measurements as follows: HOMA-IR=fasting serum insulin (mU l−1) × FPG (mmol l−1)/22.5.16 BMI ⩾25.0 kg m−2 was defined as overweight/obesity according to the World Health Organization criterion.15 The diagnostic definition for Mets followed the 2007 Joint Committee for Developing Chinese Guidelines on prevention and treatment of dyslipidemia definition,17 which is the presence of three or more of the following: (1) central obesity of W >90 cm in men or >85 cm in women; (2) TG level of ⩾1.7 mmol l−1 or specific treatment for lipid abnormality; (3) HDL-c <1.04 mmol l−1 (4) systolic BP (SBP) ⩾130 mmHg and/or diastolic BP (DBP) ⩾85 mmHg or treatment of previously diagnosed hypertension and (5) hyperglycemia of FPG ⩾6.1 mmol l−1 and/or 2hPG level ⩾7.8 mmol l−1 or previously diagnosed type 2 diabetes.
Carotid artery measurement
Carotid artery scans were performed using a high-resolution B-mode scanner (Sequoia 512, Siemens, Bonn, Germany) and a 10-MHz probe as previously described.18 A single sonographer who was blinded to clinical characteristics measured C-IMT. Both common carotid arteries were scanned from proximal to distal to the bifurcation. C-IMT was measured at the far wall of both common carotid arteries approximately 1 cm proximal to the carotid bulb. Carotid IMT was defined as the mean of the maximal IMT of each carotid artery.
Magnetic resonance imaging
Visceral and subcutaneous adipose tissue areas were assessed using a 3.0. T clinical MRI scanner (Archiva, Philips Medical System, Amsterdam, The Netherlands), using the abdominal coil. MRI scans were obtained at the abdominal level between L4 and L5 verterbrae in the supine position. Segmentation of the images into VFA and subcutaneous fat area (SFA) were performed by two trained observers using SLICE-O-MATIC image analysis software (version 4.2; Tomovision Inc., Montreal, QC, Canada). If results differed by more than 10%, a third observer who did not know the results reanalyzed the images.
Statistical analysis
All statistical analysis was performed with Statistical Package for Social Sciences version 13.0. (SPSS, Chicago, IL, USA). Data are presented as mean±standard deivation (s.d.) except for skewed variables, which are presented as median (interquartile range 25–75%). Clinical characteristics that followed a normal distribution were compared among the four groups using an unpaired Student's t-test, and those that were not normally distributed were compared with a Wilcoxon rank-sum test. For dichotomous or categorical variables, comparison between groups was performed using a χ2 test. Partial correlation analysis was performed to investigate the association between C-IMT and other parameters adjusted for anti-hypertensive, anti-diabetic and lipid-lowering treatment. Multiple stepwise regression analysis was applied to assess associations between C-IMT, VFA and metabolic parameters after adjusting for potential confounders. All reported P values were two-tailed, and P-values <0.05 were considered statistically significant.
Results
Clinical characteristics of subjects
The final dataset included 1005 subjects (515 men, 240 premenopausal women and 250 postmenopausal women) aged 34–66 years (50.7±6.8 years). The principle characteristics of the study subjects are in Table 1. Compared with men, both premenopausal and postmenopausal women had significantly lower W, VFA, C-IMT, TG and FPG, and higher %fat, HDL-c and SFA (all P<0.05). Postmenopausal women also had significantly higher age, TC, LDL-c and HbA1c than men and premenopausal women (all P<0.01). The frequency of Mets was significantly increased in men and the proportion of current smokers showed the same trend for both genders (all P<0.05).
Relationship between VFA and C-IMT by BMI category
Overweight/obese subjects (BMI ⩾25.0 kg m−2) when compared with lean subjects had a higher W, %fat, SFA, VFA, BP, CRP and C-IMT (all P<0.01). They also had a worse glucose tolerance status (FPG, 2hPG, HbA1c, HOMA-IR) and lipid profile (all P<0.05). The prevalence of Mets and its components was higher in the BMI ⩾25.0 kg m−2 category (P<0.01). However, no difference in age or smoking rate was observed between overweight/obese and lean subjects (Table 2).
To analyze the influence of visceral obesity on C-IMT by BMI category, subjects were divided into subgroups according to VFA (Table 2). In addition to increased CVD risk factors, C-IMT was elevated as VFA increased in both the BMI <25.0 kg m−2 group and the BMI ⩾25.0 kg m−2 group (for BMI <25.0 kg m−2, C-IMT was 0.60 mm (0.60–0.65) vs 0.60 mm (0.6–0.70), P<0.01 and for BMI ⩾25.0 kg m−2, C-IMT was 0.60 mm (0.60–0.65) vs 0.65 mm (0.60–0.70), P<0.01).
Association of C-IMT with anthropometric parameters, glucose, lipid profile and VFA
Table 3 shows partial correlation analysis adjusted for anti-hypertensive, anti-diabetic and lipid-lowering treatment for participants in different BMI categories. C-IMT showed significant positive correlation with age, W, VFA, SBP, LDL-c and 2hPG, and negative correlation with %fat in both the BMI <25.0 kg m−2 group and the BMI ⩾25.0 kg m−2 group (all P<0.05). In addition, C-IMT was positively correlated with DBP, TC, FPG, HbA1c and HOMA-IR in the BMI <25.0 kg m−2 group (all P<0.05). It was positively correlated with serum CRP and inversely correlated with SFA and HDL-c in the BMI ⩾25.0 kg m−2 group (all P<0.05).
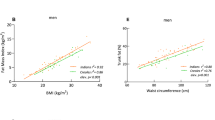
To further investigate the independent association between visceral obesity and C-IMT, multiple stepwise regression analysis was performed in men and women (Table 4). As independent variables, we used body fat parameters, the basic CVD risk factors identified in the univariate analysis of age, BP, HOMA-IR, 2hPG, LDL-c, HDL-c and CRP, as well as smoking status, anti-hypertensive therapy, anti-diabetic therapy and lipid-lowering therapy. The analysis adjusted for menopausal status in women. Three regression models were constructed according to the selected body fat parameters. The first model included all body fat parameters, including anthropometric variables (W and BMI) and accurate adiposity variables (%fat, VFA and SFA). We found that in addition to age, smoking, LDL-c, SBP and 2hPG, W (standardized β=0.163, P<0.001) was an independent factor for C-IMT in men. We next analyzed the strength of anthropometric obesity variables in model 2 and accurate adiposity variables in model 3. W (standardized β=0.161, P<0.001) and VFA (standardized β=0.107, P=0.007) were found to be two obesity-related factors significantly influencing C-IMT, respectively, in these two models in men. However, in women, no body fat parameters, except for age, HOMA-IR, SBP and anti-hypertensive therapy, affected the three models.
Discussion
Obesity is closely related to impaired glucose tolerance, dyslipidemia, hypertension and other cardiovascular risk factors, as well as cardiovascular events.19, 20, 21 As abdominal obesity might be more atherogenic than general obesity,22, 23 measurement of visceral abdominal tissue could be an early detection method for Mets and a strategic screening to prevent CVD. However, the optimal cutoff for VFA to identify early-stage atherosclerosis is still uncertain. Several groups proposed a cutoff of VFA 100 cm2 to define abdominal obesity and predict obesity-related disorders.24 In our previous study, VFA ⩾80 cm2 was found to be the discriminating value for predicting Mets in a Chinese population.12 Here, we found that individuals with VFA ⩾80 cm2 had a significantly higher incidence of Mets, in both the lean category (BMI <25.0 kg m−2) and the overweight/obesity category (BMI ⩾25.0 kg m−2). This supports this VFA value as an optimal cutoff point for identifying individuals in a Chinese population with metabolic risk factors that are not solely dependent on BMI.
No study has focused on the relationship between VFA and C-IMT in different BMI categories in a Chinese population. In this study, we compared C-IMT between subjects with or without visceral obesity by BMI category. Subjects with VFA ⩾80 cm2 had a higher C-IMT, independent of BMI. Furthermore, we found that VFA significantly positively correlated with C-IMT in correlation analysis. Regional fat distribution differs by gender. Women generally have more subcutaneous fat than men, whereas men have more visceral fat.25 We also observed significantly higher VFA and lower %fat and SFA in men than in women, although the mean BMI in men and postmenopausal women were similar. As postmenopausal women have a higher risk of developing atherosclerosis because of loss of protection from estrogen,26 we performed separate multiple stepwise regression analyses for men and women, adjusting for menopausal status in women. We found that obesity indices reflecting visceral rather than general obesity were associated with C-IMT, mainly in men. Although the correlation weakened after adjustment for other traditional cardiovascular risk factors and other obesity indices, W and VFA were still independent risk factors for increased C-IMT in men. These results might be explained by an association between adiposity and subclinical atherosclerosis mediated by CVD risk factors that are likely to be in a causal pathway from visceral fat accumulation to CVD.22, 27 As all the obesity measures of BMI, W, %fat, VFA and SFA were highly correlated with each other, the attenuation of associations by multivariate adjustment is understandable.28 Our findings are consistent with several studies that also reported a positive correlation of C-IMT to visceral fat accumulation.29, 30
Previous publications describe a possible role for visceral fat accumulation in atherosclerosis development. Ryuichi et al.31 found a graded and independent association between visceral obesity evaluated by B-mode ultrasonography and C-IMT in subjects aged ⩾50 years with a BMI ⩾23.0 kg m−2. Kim et al.,32 found that visceral fat amount was associated with carotid atherosclerosis in type 2 diabetic men with a normal W. However, we could not compare these findings directly with ours because of the indirect measurement of visceral fat and differences in study subjects. Our investigation suggested that subjects defined as not obese by BMI had increased C-IMT because some individuals were prone to visceral fat accumulation for a given BMI. Some people with higher BMI but a lower quantity of visceral fat might be categorized as high-risk obese subjects, even though they actually have a lower C-IMT and are at a low risk for metabolic complications.
Although a cause–effect relationship has not been established, possible mechanisms responsible for the relationship between visceral fat accumulation and subclinical atherosclerosis are as follows. Obesity is considered to be a chronic inflammation state, in which the excess accumulation of visceral adipose tissue has a central role.33 In this study, we also observed that CRP, a plasma marker of chronic low-grade inflammation, was significantly increased in subjects with abdominal obesity and correlated with C-IMT. However, this association was abolished in multivariable regression analysis after adjustment for parameters related to adiposity, insulin resistance, glucose and lipid metabolism. A possible explanation is that traditional cardiovascular risk factors display stronger pro-atherosclerotic effects than CRP. It is also possible that the relationship between CRP and atherosclerosis is mediated by abdominal obesity. In addition, visceral fat accumulation may be related to insulin resistance of the liver.34 East Asians are prone to have more visceral fat than people of European descent with the same BMI.35 Our results further demonstrate that the Chinese men show a greater propensity to develop CVD at a relatively low BMI.
The limitations of this study included the study population of primarily middle-aged Chinese, limiting the generalizability of our findings to other ethnic and age groups. The sample size of women, especially postmenopausal women, might not have been large enough for us to detect the contribution of visceral obesity to carotid atherosclerosis. Large population-based studies are required to confirm this association. Furthermore, the cross-sectional design precluded any inference of a casual relation between VFA, BMI and C-IMT. Future cardiovascular events and mortality should be recorded in well-controlled, prospective studies.
In conclusion, we investigated the association between VFA and C-IMT stratified by BMI. VFA positively correlated with C-IMT in a middle-aged Chinese population. VFA ⩾80 cm2 was effective for identifying carotid atherosclerosis for both lean and generally obese men.
References
Eckel RH, Krauss RM . American Heart Association call to action: obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation 1998; 97: 2099–2100.
Koskinen J, Kähönen M, Viikari JS, Taittonen L, Laitinen T, Rönnemaa T et al. Conventional cardiovascular risk factors and metabolic syndrome in predicting carotid intima-media thickness progression in young adults: the cardiovascular risk in young Finns study. Circulation 2009; 120: 229–236.
Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 2007; 92: 2240–2247.
Yu RH, Ho SC, Ho SS, Woo JL, Ahuja AT . Association of general and abdominal obesities and metabolic syndrome with subclinical atherosclerosis in asymptomatic Chinese postmenopausal women. Menopause 2008; 15: 185–192.
Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med 1998; 158: 1855–1867.
Vigili de Kreutzenberg S, Kiwanuka E, Tiengo A, Avogaro A . Visceral obesity is characterized by impaired nitric oxide-independent vasodilation. Eur Heart J 2003; 24: 1210–1215.
Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S . Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol 2001; 88: 1264–1269.
Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 2000; 102: 179–184.
Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S . Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987; 36: 54–59.
Trevisan M, Liu J, Bahsas FB, Menotti A . Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol 1998; 148: 958–966.
Alberti KG, Zimmet P, Shaw J . Metabolic syndrome--a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006; 23: 469–480.
Bao Y, Lu J, Wang C, Yang M, Li H, Zhang X et al. Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis 2008; 201: 378–384.
Grobbee DE, Bots ML . Carotid artery intima-media thickness as an indicator of generalized atherosclerosis. J Intern Med 1994; 236: 567–573.
Mitsuhashi N, Onuma T, Kubo S, Takayanagi N, Honda M, Kawamori R . Coronary artery disease and carotid artery intima-media thickness in Japanese type 2 diabetic patients. Diabetes Care 2002; 25: 1308–1312.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: 1–253.
Mattews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 2007; 35: 390–419.
Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R . Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986; 74: 1399–1406.
Després JP, Lemieux I . Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887.
Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008; 117: 605–613.
Lee CD, Jacobs Jr DR, Schreiner PJ, Iribarren C, Hankinson A . Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 2007; 86: 48–54.
Fox CS, Hwang SJ, Massaro JM, Lieb K, Vasan RS, O'Donnell CJ et al. Relation of subcutaneous and visceral adipose tissue to coronary and abdominal aortic calcium (from the Framingham Heart Study). Am J Cardiol 2009; 104: 543–547.
Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB . Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008; 117: 1658–1667.
Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66: 987–992.
Blaak E . Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 2001; 4: 499–502.
Stampfer MJ, Colditz GA, Willett WC . Menopause and heart disease: a review. Ann NY Acad Sci 1990; 592: 263–271.
Choi SY, Kim D, Oh BH, Kim M, Park HE, Lee CH et al. General and abdominal obesity and abdominal visceral fat accumulation associated with coronary artery calcification in Korean men. Atherosclerosis 2010; 213: 273–278.
Nakamura Y, Sekikawa A, Kadowaki T, Kadota A, Kadowaki S, Maegawa H et al. Visceral and subcutaneous adiposity and adiponectin in middle-aged Japanese men: the ERA JUMP study. Obesity (Silver Spring) 2009; 17: 1269–1273.
Lakka TA, Lakka HM, Salonen R, Kaplan GA, Salonen JT . Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis 2001; 154: 497–504.
De Michele M, Panico S, Iannuzzi A, Celentano E, Ciardullo AV, Galasso R et al. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke 2002; 33: 2923–2928.
Kawamoto R, Ohtsuka N, Ninomiya D, Nakamura S . Association of obesity and visceral fat distribution with intima-media thickness of carotid arteries in middle-aged and older persons. Intern Med 2008; 47: 143–149.
Kim SK, Park SW, Kim SH, Cha BS, Lee HC, Cho YW . Visceral fat amount is associated with carotid atherosclerosis even in type 2 diabetic men with a normal waist circumference. Int J Obes (Lond) 2009; 33: 131–135.
Ferroni P, Basili S, Falco A, Davi G . Inflammation, insulin resistance, and obesity. Curr Atheroscler Rep 2004; 6: 424–431.
Johnson D, Prud'homme D, Després JP, Nadeau A, Tremblay A, Bouchard C . Relation of abdominal obesity to hyperinsulinemia and high blood pressure in men. Int J Obes Relat Metab Disord 1992; 16: 881–890.
Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P . The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord 2000; 24: 1011–1017.
Acknowledgements
This work was funded by Chinese National 973 Project (2007CB914702), National Key Technology R&D Program of China (2009BAI80B01), the Shanghai United Developing Technology Project of Municipal Hospitals (SHDC12010115), Major Program of Shanghai Municipality for Basic Research (08dj1400601) and Project of National Natural Science Foundation of China (81170788).
Author contributions
Y Bao and W Jia conceived and designed the study. X Ma, Y Wang, M Zhou, W Zong and Y Hao recruited the samples. X Ma, Y Wang and M Zhou did the statistical analyses. J Zhu and D Li performed the carotid artery ultrasound scans. Y Xiao performed the MRI scans. X Ma and Y Wang wrote the first draft of the paper. X Ma, Y Wang, M Zhou, Y Bao and W Jia revised the paper and contributed to the discussion. M Zhou, L Zhang and W Zong provided the technical support. Y Wang and X Ma contributed equally to this work and are the guarantors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, Y., Ma, X., Zhou, M. et al. Contribution of visceral fat accumulation to carotid intima–media thickness in a Chinese population. Int J Obes 36, 1203–1208 (2012). https://doi.org/10.1038/ijo.2011.222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.222
Keywords
This article is cited by
-
Analysis of risk factors for carotid intima-media thickness in patients with type 2 diabetes mellitus in Western China assessed by logistic regression combined with a decision tree model
Diabetology & Metabolic Syndrome (2020)
-
Neck circumference as an effective measure for identifying cardio-metabolic syndrome: a comparison with waist circumference
Endocrine (2017)
-
Reappraisal of waist circumference cutoff value according to general obesity
Nutrition & Metabolism (2016)
-
Elevation in fibroblast growth factor 23 and its value for identifying subclinical atherosclerosis in first-degree relatives of patients with diabetes
Scientific Reports (2016)
-
Obesity in China: its characteristics, diagnostic criteria, and implications
Frontiers of Medicine (2015)