Abstract
Objective:
The skeletal muscle of obese humans is characterized by an inability to appropriately respond to alterations in substrate availability. The purpose of this study was to determine if this metabolic inflexibility with obesity is retained in mitochondria of human skeletal muscle cells raised in culture (HSkMC) and to identify potential mechanisms involved.
Design:
Mitochondrial respiration was measured in permeabilized myotubes cultured from lean and obese individuals before and after a 24-h lipid incubation.
Results:
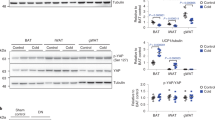
Mitochondrial respiration (state 3) in the presence of lipid substrate (palmitoyl carnitine) increased by almost twofold after lipid incubation in HSkMC from lean, but not obese subjects, indicative of metabolic inflexibility with obesity. The 24-h lipid incubation increased mitochondrial DNA (mtDNA) copy number in HSkMC from lean subjects by +16% (P<0.05); conversely, mtDNA copy number decreased in myotubes cultured from obese individuals (−13%, P=0.06). When respiration data were normalized to mtDNA copy number and other indices of mitochondrial content (COX-IV protein content and CS activity), the significant treatment effects of lipid incubation persisted in the lean subjects, suggesting concomitant alterations in mitochondrial function; no similar adjustment was evident in HSkMC from obese individuals.
Conclusion:
These data indicate that the skeletal muscle of obese individuals inherently lacks metabolic flexibility in response to lipid exposure, which consists of an inability to increase mitochondrial respiration in the presence of lipid substrate and perhaps by an inability to induce mitochondrial proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2005; 2: 251–261.
Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 2003; 284: E741–E747.
Kelley DE, Goodpaster B, Wing RR, Simoneau JA . Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 1999; 277 (6 Part 1): E1130–E1141.
Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA . Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 2000; 279: E1039–E1044.
Goodpaster BH, Theriault R, Watkins SC, Kelley DE . Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000; 49: 467–472.
Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009; 119: 573–581.
Kelley DE, He J, Menshikova EV, Ritov VB . Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002; 51: 2944–2950.
Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007; 56: 1592–1599.
Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F . Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007; 50: 790–796.
Holloway GP, Thrush AB, Heigenhauser GJ, Tandon NN, Dyck DJ, Bonen A et al. Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am J Physiol Endocrinol Metab 2007; 292: E1782–E1789.
Lefort N, Glancy B, Bowen B, Willis WT, Bailowitz Z, De Filippis EA et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes 2010; 59: 2444–2452.
Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes 2008; 57: 2943–2949.
Boyle KE, Canham JP, Consitt LA, Zheng D, Koves TR, Gavin TP et al. A high-fat diet elicits differential responses in genes coordinating oxidative metabolism in skeletal muscle of lean and obese individuals. J Clin Endocrinol Metab 2011; 96: 775–781.
Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA et al. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest 2005; 115: 1934–1941.
Kelley DE, Mandarino LJ . Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 2000; 49: 677–683.
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008; 7: 45–56.
Consitt LA, Bell JA, Koves TR, Muoio DM, Hulver MW, Haynie KR et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes 2010; 59: 1407–1415.
Gaster M . Reduced lipid oxidation in myotubes established from obese and type 2 diabetic subjects. Biochem Biophys Res Commun 2009; 382: 766–770.
Wensaas AJ, Rustan AC, Just M, Berge RK, Drevon CA, Gaster M . Fatty acid incubation of myotubes from humans with type 2 diabetes leads to enhanced release of beta-oxidation products because of impaired fatty acid oxidation: effects of tetradecylthioacetic acid and eicosapentaenoic acid. Diabetes 2009; 58: 527–535.
Corpeleijn E, Hessvik NP, Bakke SS, Levin K, Blaak EE, Thoresen GH et al. Oxidation of intramyocellular lipids is dependent on mitochondrial function and the availability of extracellular fatty acids. Am J Physiol Endocrinol Metab 2010; 299: E14–E22.
Berggren JR, Tanner CJ, Houmard JA . Primary cell cultures in the study of human muscle metabolism. Exerc Sport Sci Rev 2007; 35: 56–61.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009; 458: 1056–1060.
Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 2008; 8: 347–358.
Philp A, Perez-Schindler J, Green C, Hamilton DL, Baar K . Pyruvate suppresses PGC1alpha expression and substrate utilization despite increased respiratory chain content in C2C12 myotubes. Am J Physiol Cell Physiol 2010; 299: C240–C250.
Gnaiger E . Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol 2009; 41: 1837–1845.
Baar K . Epigenetic control of skeletal muscle fibre type. Acta Physiol (Oxf) 2010; 199: 477–487.
Gaster M, Petersen I, Hojlund K, Poulsen P, Beck-Nielsen H . The diabetic phenotype is conserved in myotubes established from diabetic subjects: evidence for primary defects in glucose transport and glycogen synthase activity. Diabetes 2002; 51: 921–927.
Cameron-Smith D, Burke LM, Angus DJ, Tunstall RJ, Cox GR, Bonen A et al. A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am J Clin Nutr 2003; 77: 313–318.
Peters SJ, Harris RA, Wu P, Pehleman TL, Heigenhauser GJ, Spriet LL . Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet. Am J Physiol Endocrinol Metab 2001; 281: E1151–E1158.
Astrup A, Buemann B, Christensen NJ, Toubro S . Failure to increase lipid oxidation in response to increasing dietary fat content in formerly obese women. Am J Physiol 1994; 266 (4 Part 1): E592–E599.
Thomas CD, Peters JC, Reed GW, Abumrad NN, Sun M, Hill JO . Nutrient balance and energy expenditure during ad libitum feeding of high-fat and high-carbohydrate diets in humans. Am J Clin Nutr 1992; 55: 934–942.
Gaster M . Metabolic flexibility is conserved in diabetic myotubes. J Lipid Res 2007; 48: 207–217.
Bell JA, Reed MA, Consitt LA, Martin OJ, Haynie KR, Hulver MW et al. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J Clin Endocrinol Metab 2010; 95: 3400–3410.
Kitzmann M, Lantier L, Hebrard S, Mercier J, Foretz M, Aguer C . Abnormal metabolism flexibility in response to high palmitate concentrations in myotubes derived from obese type 2 diabetic patients. Biochim Biophys Acta 2011; 1812: 423–430.
Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Moonen-Kornips E, Schaart G, Mustard KJ et al. Intramyocellular lipid content and molecular adaptations in response to a 1-week high-fat diet. Obes Res 2005; 13: 2088–2094.
Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 2005; 280: 33588–33598.
Schrauwen P . High-fat diet, muscular lipotoxicity and insulin resistance. Proc Nutr Soc 2007; 66: 33–41.
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98: 115–124.
Staiger H, Staiger K, Haas C, Weisser M, Machicao F, Haring HU . Fatty acid-induced differential regulation of the genes encoding peroxisome proliferator-activated receptor-gamma coactivator-1alpha and -1beta in human skeletal muscle cells that have been differentiated in vitro. Diabetologia 2005; 48: 2115–2118.
Acknowledgements
This work was supported by NIH Grants AG025205 and DK56112 (to JAH) and DK073488 (to PDN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Boyle, K., Zheng, D., Anderson, E. et al. Mitochondrial lipid oxidation is impaired in cultured myotubes from obese humans. Int J Obes 36, 1025–1031 (2012). https://doi.org/10.1038/ijo.2011.201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.201
Keywords
This article is cited by
-
Roux-en-Y gastric bypass surgery restores insulin-mediated glucose partitioning and mitochondrial dynamics in primary myotubes from severely obese humans
International Journal of Obesity (2020)
-
Substrate oxidation in primary human skeletal muscle cells is influenced by donor age
Cell and Tissue Research (2020)
-
Altered tricarboxylic acid cycle flux in primary myotubes from severely obese humans
International Journal of Obesity (2019)
-
Eight weeks of overfeeding alters substrate partitioning without affecting metabolic flexibility in men
International Journal of Obesity (2017)
-
Insulin resistance is associated with epigenetic and genetic regulation of mitochondrial DNA in obese humans
Clinical Epigenetics (2015)