Abstract
Background:
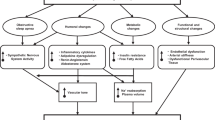
Sympathetic activation is an important metabolic adaptation limiting weight gain. Propensity of weight gain associated with β-blocker therapy in the obese modern population is unknown.
Objective:
To determine whether chronic β-blocker therapy reduces energy expenditure (EE) and increases body weight.
Methods:
We undertook (i) a mechanistic study comparing EE, diet-induced thermogenesis and habitual activity between healthy volunteers (n=11) with uncomplicated hypertension treated with a β-blocker and anthropometrically matched controls (n=19) and (ii) three cross-sectional studies comparing body weight, body mass index (BMI) and waist circumference between β-blocker treated and untreated patients from ambulatory patients attending (a) diabetes outpatient clinic (n=214), (b) hypertension outpatient (n=84) and (c) participants in a multi-centre type 2 diabetes trial (ADVANCE) (n=11140).
Results:
Among weight-matched β-blocker users, diet-induced thermogenesis, fat oxidation rate and weekly habitual activity were lower by 50% (P<0.01), 32% (P=0.04) and 30% (P<0.01), respectively, compared with controls. In β-blocker treated patients, the adjusted mean body weight was 9.2±1.2 kg (P=0.0002) higher among those attending the diabetes clinic, 17.2±3.2 kg (P=0.004) higher among those attending the hypertension clinic and 5.2±0.7 kg (P=0.0003) higher at baseline among participants in the ADVANCE trial compared with patients not treated with β-blockers. BMI displayed a similar difference.
Conclusions:
EE is reduced and body weight increased in chronic β-blocker users. We hypothesise that chronic β-blockade causes obesity by blunting EE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096.
Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA 2005; 293: 1868–1874.
Flegal KM, Carroll MD, Ogden CL, Curtin LR . Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–241.
Zaninotto P, Head J, Stamatakis E, Wardle H, Mindell J . Trends in obesity among adults in England from 1993 to 2004 by age and social class and projections of prevalence to 2012. J Epidemiol Community Health 2009; 63: 140–146.
Greenfield JR, Campbell LV . Role of the autonomic nervous system and neuropeptides in the development of obesity in humans: targets for therapy? Curr Pharm Des 2008; 14: 1815–1820.
Bray GA . Obesity, a disorder of nutrient partitioning: the MONA LISA hypothesis. J Nutr 1991; 121: 1146–1162.
Bray GA . Obesity--a state of reduced sympathetic activity and normal or high adrenal activity (the autonomic and adrenal hypothesis revisited). Int J Obes 1990; 14 (Suppl 3): 77–91; discussion 91–72.
Astrup A . The sympathetic nervous system as a target for intervention in obesity. Int J Obes Relat Metab Disord 1995; 19 (Suppl 7): S24–S28.
Macdonald IA . Advances in our understanding of the role of the sympathetic nervous system in obesity. Int J Obes Relat Metab Disord 1995; 19 (Suppl 7): S2–S7.
Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 2002; 297: 843–845.
Buemann B, Astrup A, Quaade F, Madsen J . 24-h energy expenditure and substrate oxidation rates are unaffected by body fat distribution in obese women. Metabolism 1994; 43: 109–113.
Acheson K, Jequier E, Wahren J . Influence of beta-adrenergic blockade on glucose-induced thermogenesis in man. J Clin Invest 1983; 72: 981–986.
Lamont LS, Brown T, Riebe D, Caldwell M . The major components of human energy balance during chronic beta-adrenergic blockade. J Cardiopulm Rehabil 2000; 20: 247–250.
Gordon NF, van Rensburg JP, van den Heever DP, Kalliatakis NB, Myburgh DP . Effect of dual beta-blockade and calcium antagonism on endurance performance. Med Sci Sports Exerc 1987; 19: 1–6.
Dressendorfer RH, Franklin BA, Gordon S, Timmis GC . Resting oxygen uptake in coronary artery disease. Influence of chronic beta-blockade. Chest 1993; 104: 1269–1272.
Steinkraus V, Nose M, Scholz H, Thormahlen K . Time course and extent of alpha 1-adrenoceptor density changes in rat heart after beta-adrenoceptor blockade. Br J Pharmacol 1989; 96: 441–449.
Study rationale and design of ADVANCE: action in diabetes and vascular disease--preterax and diamicron MR controlled evaluation. Diabetologia 2001; 44: 1118–1120.
Merlo J, Wessling A, Melander A . Comparison of dose standard units for drug utilisation studies. Eur J Clin Pharmacol 1996; 50: 27–30.
O’Sullivan AJ, Crampton LJ, Freund J, Ho KK . The route of estrogen replacement therapy confers divergent effects on substrate oxidation and body composition in postmenopausal women. J Clin Invest 1998; 102: 1035–1040.
Frayn KN . Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983; 55: 628–634.
Ferrannini E . The theoretical bases of indirect calorimetry: a review. Metabolism 1988; 37: 287–301.
Greenfield JR, Samaras K, Hayward CS, Chisholm DJ, Campbell LV . Beneficial postprandial effect of a small amount of alcohol on diabetes and cardiovascular risk factors: modification by insulin resistance. J Clin Endocrinol Metab 2005; 90: 661–672.
O’Sullivan AJ, Kelly JJ, Hoffman DM, Freund J, Ho KK . Body composition and energy expenditure in acromegaly. J Clin Endocrinol Metab 1994; 78: 381–386.
Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ . Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 1996; 45: 633–638.
Richardson MT, Ainsworth BE, Jacobs DR, Leon AS . Validation of the Stanford 7-day recall to assess habitual physical activity. Ann Epidemiol 2001; 11: 145–153.
Rowe DA, Kemble CD, Robinson TS, Mahar MT . Daily walking in older adults: day-to-day variability and criterion-referenced validity of total daily step counts. J Phys Act Health 2007; 4: 434–446.
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high Blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497.
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Lee P, Greenfield JR, Ho KK, Fulham MJ . A critical appraisal of prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010; 299: E601–E606.
Soderlund V, Larsson SA, Jacobsson H . Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging 2007; 34: 1018–1022.
Sharma AM, Pischon T, Hardt S, Kunz I, Luft FC . Hypothesis: Beta-adrenergic receptor blockers and weight gain: A systematic analysis. Hypertension 2001; 37: 250–254.
Leslie WS, Hankey CR, Lean ME . Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM 2007; 100: 395–404.
Rossner S, Taylor CL, Byington RP, Furberg CD . Long term propranolol treatment and changes in body weight after myocardial infarction. BMJ 1990; 300: 902–903.
Hypertension in Diabetes Study. III. Prospective study of therapy of hypertension in type 2 diabetic patients: efficacy of ACE inhibition and beta-blockade. Diabet Med 1994; 11: 773–782.
Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ 1998; 317: 713–720.
Schiffrin EL . Correction of remodeling and function of small arteries in human hypertension by cilazapril, an angiotensin I-converting enzyme inhibitor. J Cardiovasc Pharmacol 1996; 27 (Suppl 2): S13–S18.
Foss OP, Jensen EK . The effect of captopril and metoprolol as monotherapy or combined with bendroflumethiazide on blood lipids. J Intern Med 1990; 227: 119–123.
Houston MC, Olafsson L, Burger MC . Effects of nifedipine GITS and atenolol monotherapy on serum lipids, blood pressure, heart rate, and weight in mild to moderate hypertension. Angiology 1991; 42: 681–690.
Wikstrand J, Warnold I, Olsson G, Tuomilehto J, Elmfeldt D, Berglund G . Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA 1988; 259: 1976–1982.
Wilhelmsen L, Berglund G, Elmfeldt D, Fitzsimons T, Holzgreve H, Hosie J et al. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. J Hypertens 1987; 5: 561–572.
Centers for Disease Control and Prevention (CDC). Trends in intake of energy and macronutrients--United States, 1971–2000. MMWR Morb Mortal Wkly Rep 2004; 53: 80–82.
Brownson RC, Boehmer TK, Luke DA . Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health 2005; 26: 421–443.
Cameron AJ, Welborn TA, Zimmet PZ, Dunstan DW, Owen N, Salmon J et al. Overweight and obesity in Australia: the 1999–2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med J Aust 2003; 178: 427–432.
Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL . Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 1998; 22: 39–47.
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000; 32: S498–S504.
Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J . Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab 2008; 19: 130–137.
Arner P, Kriegholm E, Engfeldt P, Bolinder J . Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest 1990; 85: 893–898.
The treatment of mild hypertension study. A randomized, placebo-controlled trial of a nutritional-hygienic regimen along with various drug monotherapies. The treatment of mild hypertension research group. Arch Intern Med 1991; 151: 1413–1423.
Davis BR, Oberman A, Blaufox MD, Wassertheil-Smoller S, Hawkins CM, Cutler JA et al. Effect of antihypertensive therapy on weight loss. The trial of antihypertensive interventions and management research group. Hypertension 1992; 19: 393–399.
Jequier E, Tappy L . Regulation of body weight in humans. Physiol Rev 1999; 79: 451–480.
Acknowledgements
We thank Wendy Bryant for the provision of data from Diabetes Clinic, St Vincent's Hospital, Sydney, Australia. We also thank the physicians in the Diabetes and Renal Hypertension Clinics for collection of data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr John Chalmers has received research grants for the ADVANCE trial from Servier International, administered through the University of Sydney. Dr Paul Lee was funded by an Australian National Health Medical Research Council postgraduate scholarship. Dr Kengne, Dr Greenfield, Dr Day and Dr Ho declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, P., Kengne, AP., Greenfield, J. et al. Metabolic sequelae of β-blocker therapy: weighing in on the obesity epidemic?. Int J Obes 35, 1395–1403 (2011). https://doi.org/10.1038/ijo.2010.284
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.284
Keywords
This article is cited by
-
Using genetics to assess the association of commonly used antihypertensive drugs with diabetes, glycaemic traits and lipids: a trans-ancestry Mendelian randomisation study
Diabetologia (2022)
-
Dysfonctions du système nerveux sympathique et de la signalisation catécholaminergique chez l’obèse
Obésité (2014)
-
Formoterol, a highly β2-selective agonist, increases energy expenditure and fat utilisation in men
International Journal of Obesity (2013)
-
A sympathetic view of human obesity
Clinical Autonomic Research (2013)