Abstract
Objective:
Accumulation of adipose tissue macrophages (ATMs) is observed in obesity and may participate in the development of insulin resistance and obesity-related complications. The aim of our study was to investigate the effect of long-term dietary intervention on ATM content in human adipose tissue.
Design:
We performed a multi-phase longitudinal study.
Subjects and measurements:
A total of 27 obese pre-menopausal women (age 39±2 years, body mass index 33.7±0.5 kg m–2) underwent a 6-month dietary intervention consisting of two periods: 4 weeks of very low-calorie diet (VLCD) followed by weight stabilization composed of 2 months of low-calorie diet and 3to 4 months of weight maintenance diet. At baseline and at the end of each dietary period, samples of subcutaneous adipose tissue (SAT) were obtained by needle biopsy and blood samples were drawn. ATMs were determined by flow cytometry using combinations of cell surface markers. Selected cytokine and chemokine plasma levels were measured using enzyme-linked immunosorbent assay. In addition, in a subgroup of 16 subjects, gene expression profiling of macrophage markers in SAT was performed using real-time PCR.
Results:
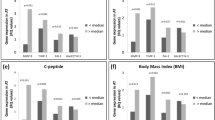
Dietary intervention led to a significant decrease in body weight, plasma insulin and C-reactive protein levels. After VLCD, ATM content defined by CD45+/14+/206+ did not change, whereas it decreased at the end of the intervention. This decrease was associated with a downregulation of macrophage marker mRNA levels (CD14, CD163, CD68 and LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1)) and plasma levels of monocyte-chemoattractant protein-1 (MCP-1) and CXCL5 (chemokine (C-X-C motif) ligand 5). During the whole dietary intervention, the proportion of two ATM subpopulations distinguished by the CD16 marker was not changed.
Conclusion:
A 6-month weight-reducing dietary intervention, but not VLCD, promotes a decrease in the number of the whole ATM population with no change in the relative distribution of ATM subsets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005; 54: 2277–2286.
Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P et al. Interplay between human adipocytes and T lymphocytes in obesity. CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol 2009; 29: 1608–1614.
Elgazar-Carmon V, Rudich A, Hadad N, Levy R . Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 2008; 49: 1894–1903.
Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008; 28: 1304–1310.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830.
Heilbronn LK, Campbell LV . Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 2008; 14: 1225–1230.
Hotamisligil GS . Inflammation and metabolic disorders. Nature 2006; 444: 860–867.
Poitou C, Coussieu C, Rouault C, Coupaye M, Cancello R, Bedel JF et al. Serum amyloid A: a marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity (Silver Spring) 2006; 14: 309–318.
Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 2008; 117: 806–815.
Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW . Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004; 145: 2273–2282.
Maury E, Ehala-Aleksejev K, Guiot Y, Detry R, Vandenhooft A, Brichard SM . Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab 2007; 293: E656–E665.
Shoelson SE, Herrero L, Naaz A . Obesity, inflammation, and insulin resistance. Gastroenterology 2007; 132: 2169–2180.
Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006; 55: 1554–1561.
Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005; 46: 2347–2355.
Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante Jr AW et al. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes 2009; 58: 385–393.
Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116: 1494–1505.
Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006; 116: 115–124.
Lumeng CN, Deyoung SM, Saltiel AR . Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab 2007; 292: E166–E174.
Nguyen MT, Favelyukis S, Nguyen AK, Reichart DD, Scott PA, Jenn A et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 2007; 282: 35279–35292.
Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol 2008; 28: 1654–1659.
Cipolletta C, Ryan KE, Hanna EV, Trimble ER . Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes 2005; 54: 2779–2786.
Tordjman J, Poitou C, Hugol D, Bouillot JL, Basdevant A, Bedossa P et al. Association between omental adipose tissue macrophages and liver histopathology in morbid obesity: influence of glycemic status. J Hepatol 2009; 51: 354–362.
Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes 2004; 53: 1285–1292.
Mosser DM, Edwards JP . Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958–969.
Stout RD, Suttles J . Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol 2004; 76: 509–513.
Lumeng CN, Bodzin JL, Saltiel AR . Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184.
Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR . Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008; 57: 3239–3246.
Bourlier V, Bouloumie A . Role of macrophage tissue infiltration in obesity and insulin resistance. Diabetes Metab 2009; 35: 251–260.
Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007; 31: 1420–1428.
Klimcakova E, Polak J, Moro C, Hejnova J, Majercik M, Viguerie N et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab 2006; 91: 5107–5112.
Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A . Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004; 110: 349–355.
Capel F, Klimcakova E, Viguerie N, Roussel B, Vitkova M, Kovacikova M et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes 2009; 58: 1558–1567.
Chavey C, Lazennec G, Lagarrigue S, Clape C, Iankova I, Teyssier J et al. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab 2009; 9: 339–349.
Conti P, DiGioacchino M . MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc 2001; 22: 133–137.
Walz A, Schmutz P, Mueller C, Schnyder-Candrian S . Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease. J Leukoc Biol 1997; 62: 604–611.
Klimcakova E, Kovacikova M, Stich V, Langin D . Adipokines and dietary interventions in human obesity. Obes Rev 2010 (e-pub ahead of print).
Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 1997; 82: 4196–4200.
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11: 191–198.
Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 2009; 296: E1300–E1310.
Suganami T, Nishida J, Ogawa Y . A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 2005; 25: 2062–2068.
Surmi BK, Hasty AH . Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol 2008; 3: 545–556.
Gordon S . Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M . The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–686.
Martinez FO, Sica A, Mantovani A, Locati M . Macrophage activation and polarization. Front Biosci 2008; 13: 453–461.
Geissmann F, Jung S, Littman DR . Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71–82.
Rogacev KS, Ulrich C, Blomer L, Hornof F, Oster K, Ziegelin M et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur Heart J 2010; 31: 369–376.
Ziegler-Heitbrock L . The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 2007; 81: 584–592.
McGrath MS, Kodelja V . Balanced macrophage activation hypothesis: a biological model for development of drugs targeted at macrophage functional states. Pathobiology 1999; 67: 277–281.
Zeyda M, Stulnig TM . Adipose tissue macrophages. Immunol Lett 2007; 112: 61–67.
Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A et al. Human adipose tissue macrophages: M1 and M2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 2009; 94: 4619–4623.
Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 2007; 6: 137–143.
Deguchi JO, Yamazaki H, Aikawa E, Aikawa M . Chronic hypoxia activates the Akt and beta-catenin pathways in human macrophages. Arterioscler Thromb Vasc Biol 2009; 29: 1664–1670.
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007; 447: 1116–1120.
Acknowledgements
We thank Zuzana Parizkova for her excellent technical assistance and Dr Zdenka Prochazkova for laboratory analysis. This work was supported by Grant IGA NS 10519-3-2009 of Ministry of Health of the Czech Republic, by research project of MSMT of the Czech Republic MSM 0021620814, by INSERM and by the Commission of the European Communities (Integrated Project HEPADIP (http://www.hepadip.org), Contract LSHM-CT-2005-018734, and Collaborative Project ADAPT (http://www.adapt-eu.net), Contract HEALTH F2-2008-2011 00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kováčiková, M., Sengenes, C., Kováčová, Z. et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int J Obes 35, 91–98 (2011). https://doi.org/10.1038/ijo.2010.112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.112
Keywords
This article is cited by
-
Alterations in the mammary gland and tumor microenvironment of formerly obese mice
BMC Cancer (2023)
-
Immune and non-immune functions of adipose tissue leukocytes
Nature Reviews Immunology (2022)
-
Weighing the Risk: effects of Obesity on the Mammary Gland and Breast Cancer Risk
Journal of Mammary Gland Biology and Neoplasia (2020)
-
Serum oxLDL–β2GPI complex reflects metabolic syndrome and inflammation in adipose tissue in obese
International Journal of Obesity (2018)
-
Regulation of immunometabolism in adipose tissue
Seminars in Immunopathology (2018)