Abstract
Context:
Very limited information is available regarding the function of human thyroid hormone responsive Spot 14 (human S14, hS14) in adipogenesis and human adiposity.
Objective:
To evaluate hS14 levels during differentiation of human pre-adipocytes, in human fat depots and isolated fat cells.
Design:
This was a cross-sectional study.
Subjects:
A total of 161 omental (OM) and 87 subcutaneous (SC) adipose tissue samples obtained during elective surgical procedures from a population who varied widely in terms of obesity.
Measurements:
hS14 gene expression and protein levels during adipogenesis were assessed by RT–PCR, western blot, and using an automated confocal imaging approach.
Results:
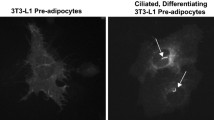
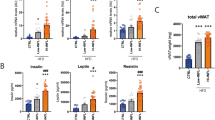
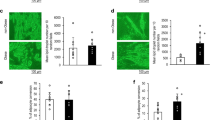
hS14 gene expression levels were decreased in OM adipose tissue from overweight (−42.0%) and obese subjects (−56.5%) compared with lean subjects (P<0.05 and P<0.0001, respectively). hS14 mRNA (but not hS14-related) was inversely associated with obesity measures such as body mass index (P=0.001), percent fat mass (P=0.001), waist-to-hip ratio (P=0.020), and systolic blood pressure (P=0.031). hS14 gene expression and protein levels were up-regulated at the early stages of differentiation of human pre-adipocytes as well as for 3T3-L1 cells. That observation was most prominent in those individual cells exhibiting the more marked differentiation features. hS14 gene expression levels increased by ∼45 000-fold in mature adipocytes. Increased hS14 levels were also found in stromal-vascular cells/pre-adipocytes (3.8-fold, P<0.05) and in adipose tissue samples (1.9-fold, P<0.0001) from SC compared with OM fat depots.
Conclusions:
These results suggest that hS14 is involved in human adipogenesis, but inversely related to obesity and OM fat accumulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hillgartner FB, Salati LM, Goodridge AG . Physiological and molecular mechanisms involved in nutritional regulation of fatty acid synthesis. Physiol Rev 1995; 75: 47–76.
Girard J, Ferre P, Foufelle F . Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu Rev Nutr 1997; 17: 325–352.
Towle HC, Kaytor EN, Shih HM . Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu Rev Nutr 1997; 17: 405–433.
Vaulont S, Vasseur-Cognet M, Kahn A . Glucose regulation of gene transcription. J Biol Chem 2000; 275: 31555–31558.
Iritani N . Nutritional and hormonal regulation of lipogenic-enzyme gene expression in rat liver. Eur J Biochem 1992; 205: 433–442.
Claycombe KJ, Jones BH, Standridge MK, Guo Y, Chun JT, Taylor JW et al. Insulin increases fatty acid synthase gene transcription in human adipocytes. Am J Physiol 1998; 274: R1253–R1259.
Kim KH . Regulation of mammalian acetyl-coenzyme A carboxylase. Annu Rev Nutr 1997; 17: 77–99.
Freake HC, Moon YK . Hormonal and nutritional regulation of lipogenic enzyme mRNA levels in rat primary white and brown adipocytes. J Nutr Sci Vitaminol (Tokyo) 2003; 49: 40–46.
Kinlaw WB, Tron P, Friedmann AS . Nuclear localization and hepatic zonation of rat ‘spot 14’ protein: immunohistochemical investigation employing anti-fusion protein antibodies. Endocrinology 1992; 131: 3120–3122.
Martel PM, Bingham CM, McGraw CJ, Baker CL, Morganelli PM, Meng ML et al. S14 protein in breast cancer cells: direct evidence of regulation by SREBP-1c, superinduction with progestin, and effects on cell growth. Exp Cell Res 2006; 312: 278–288.
Mariash CN, Seelig S, Schwartz HL, Oppenheimer JH . Rapid synergistic interaction between thyroid hormone and carbohydrate on mRNAS14 induction. J Biol Chem 1986; 261: 9583–9586.
Jump DB, Bell A, Santiago V . Thyroid hormone and dietary carbohydrate interact to regulate rat liver S14 gene transcription and chromatin structure. J Biol Chem 1990; 265: 3474–3478.
Narayan P, Liaw CW, Towle HC . Rapid induction of a specific nuclear mRNA precursor by thyroid hormone. Proc Natl Acad Sci USA 1984; 81: 4687–4691.
Shih HM, Liu Z, Towle HC . Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J Biol Chem 1995; 270: 21991–21997.
Liu HC, Towle HC . Functional synergism between multiple thyroid hormone response elements regulates hepatic expression of the rat S14 gene. Mol Endocrinol 1994; 8: 1021–1037.
Perez-Castillo A, Schwartz HL, Oppenheimer JH . Rat hepatic mRNA-S14 and lipogenic enzymes during weaning: role of S14 in lipogenesis. Am J Physiol 1987; 253: E536–E542.
Jump DB, Clarke SD, MacDougald O, Thelen A . Polyunsaturated fatty acids inhibit S14 gene transcription in rat liver and cultured hepatocytes. Proc Natl Acad Sci USA 1993; 90: 8454–8458.
Kinlaw WB, Church JL, Harmon J, Mariash CN . Direct evidence for a role of the ‘spot 14’ protein in the regulation of lipid synthesis. J Biol Chem 1995; 270: 16615–16618.
Zhu Q, Anderson GW, Mucha GT, Parks EJ, Metkowski JK, Mariash CN . The Spot 14 protein is required for de novo lipid synthesis in the lactating mammary gland. Endocrinology 2005; 146: 3343–3350.
Tsatsos NG, Augustin LB, Anderson GW, Towle HC, Mariash CN . Hepatic expression of the SPOT 14 (S14) paralog S14-related (Mid1 interacting protein) is regulated by dietary carbohydrate. Endocrinology 2008; 149: 5155–5161.
Anderson GW, Zhu Q, Metkowski J, Stack MJ, Gopinath S, Mariash CN . The Thrsp null mouse (Thrsp(tm1cnm)) and diet-induced obesity. Mol Cell Endocrinol 2009; 302: 99–107.
Moncur JT, Park JP, Maloney M, Mohandas TK, Kinlaw WB . Assignment of the ‘spot 14’ gene (THRSP) to human chromosome band 11q13.5 by in situ hybridization. Cytogenet Cell Genet 1997; 78: 131–132.
Grillasca JP, Gastaldi M, Khiri H, Dace A, Peyrol N, Reynier P et al. Cloning and initial characterization of human and mouse Spot 14 genes. FEBS Lett 1997; 401: 38–42.
Ota Y, Mariash A, Wagner JL, Mariash CN . Cloning, expression and regulation of the human S14 gene. Mol Cell Endocrinol 1997; 126: 75–81.
Kirschner LS, Mariash CN . Adipose S14 mRNA is abnormally regulated in obese subjects. Thyroid 1999; 9: 143–148.
Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C . Adipose-derived stem cells: isolation, expansion and differentiation. Methods 2008; 45: 115–120.
Deurenberg P, van der Kooy K, Leenen R, Weststrate JA, Seidell JC . Sex and age specific prediction formulas for estimating body composition from bioelectrical impedance: a cross-validation study. Int J Obes 1991; 15: 17–25.
Fernandez-Real JM, Broch M, Ricart W, Casamitjana R, Gutierrez C, Vendrell J et al. Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes 1998; 47: 1757–1762.
Ortega FJ, Moreno-Navarrete JM, Ribas V, Esteve E, Rodriguez-Hermosa JI, Ruiz B et al. Subcutaneous fat shows higher thyroid hormone receptor-alpha1 gene expression than omental fat. Obesity (Silver Spring) 2009; 17: 2134–2141.
Ortega FJ, Mayas D, Moreno-Navarrete JM, Catalán V, Gómez-Ambrosi J, Esteve E et al. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 2009, e-pub ahead of print 18 June 2009, doi:10.1038/oby.2009.202.
Jump DB, Oppenheimer JH . High basal expression and 3,5,3′-triiodothyronine regulation of messenger ribonucleic acid S14 in lipogenic tissues. Endocrinology 1985; 117: 2259–2266.
Jump DB, Bell A, Lepar G, Hu D . Insulin rapidly induces rat liver S14 gene transcription. Mol Endocrinol 1990; 4: 1655–1660.
Walker JD, Burmeister LA, Mariash A, Bosman JF, Harmon J, Mariash CN . Insulin increases the processing efficiency of messenger ribonucleic acid-S14 nuclear precursor. Endocrinology 1996; 137: 2293–2299.
Brown SB, Maloney M, Kinlaw WB . ‘Spot 14’ protein functions at the pretranslational level in the regulation of hepatic metabolism by thyroid hormone and glucose. J Biol Chem 1997; 272: 2163–2166.
Kinlaw WB, Tron P, Witters LA . Thyroid hormone and dietary carbohydrate induce different hepatic zonation of both ‘spot 14’ and acetyl-coenzyme-A carboxylase: a novel mechanism of coregulation. Endocrinology 1993; 133: 645–650.
Chou WY, Cheng YS, Ho CL, Liu ST, Liu PY, Kuo CC et al. Human spot 14 protein interacts physically and functionally with the thyroid receptor. Biochem Biophys Res Commun 2007; 357: 133–138.
Chou WY, Ho CL, Tseng ML, Liu ST, Yen LC, Huang SM . Human Spot 14 protein is a p53-dependent transcriptional coactivator via the recruitment of thyroid receptor and Zac1. Int J Biochem Cell Biol 2008; 40: 1826–1834.
Menendez JA, Vazquez-Martin A, Ortega FJ, Fernandez-Real JM . Fatty acid synthase: association with insulin resistance, type 2 diabetes, and cancer. Clin Chem 2009; 55: 425–438.
Sanchez-Rodriguez J, Kaninda-Tshilumbu JP, Santos A, Perez-Castillo A . The spot 14 protein inhibits growth and induces differentiation and cell death of human MCF-7 breast cancer cells. Biochem J 2005; 390: 57–65.
Kinlaw WB, Quinn JL, Wells WA, Roser-Jones C, Moncur JT . Spot 14: a marker of aggressive breast cancer and a potential therapeutic target. Endocrinology 2006; 147: 4048–4055.
Vazquez-Martin A, Ortega-Delgado FJ, Fernandez-Real JM, Menendez JA . The tyrosine kinase receptor HER2 (erbB-2): from oncogenesis to adipogenesis. J Cell Biochem 2008; 105: 1147–1152.
Donnelly C, Olsen AM, Lewis LD, Eisenberg BL, Eastman A, Kinlaw WB . Conjugated linoleic acid (CLA) inhibits expression of the Spot 14 (THRSP) and fatty acid synthase genes and impairs the growth of human breast cancer and liposarcoma cells. Nutr Cancer 2009; 61: 114–122.
Rorive M, Letiexhe MR, Scheen AJ, Ziegler O . Obesity and type 2 diabetes. Rev Med Liege 2005; 60: 374–382.
Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD . The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci USA 2000; 97: 11371–11376.
Soukas A, Cohen P, Socci ND, Friedman JM . Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev 2000; 14: 963–980.
Liu LH, Wang XK, Hu YD, Kang JL, Wang LL, Li S . Effects of a fatty acid synthase inhibitor on adipocyte differentiation of mouse 3T3-L1 cells. Acta Pharmacol Sin 2004; 25: 1052–1057.
Poulain-Godefroy O, Lecoeur C, Pattou F, Fruhbeck G, Froguel P . Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1–R7.
Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol 2002; 282: R1286–R1296.
Acknowledgements
We greatly appreciate the technical assistance of Gerard Pardo and Oscar Rovira (Unit of Diabetes, Endocrinology and Nutrition, Institut d’Investigació Biomèdica de Girona, Hospital Universitari Dr Josep Trueta de Girona). The work of all the members of the Multidisciplinary Obesity Team of the Clínica Universitaria de Navarra is also gratefully acknowledged. This work was supported by research grants from the Ministerio de Educación y Ciencia (SAF2008-02073 and SAF2006-02354), Generalitat de Catalunya (2005SGR00947 and 2005SGR00467), and the Instituto de Salud Carlos III (ISCIIIRETIC RD06, CIBERObN).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
All authors of this manuscript have directly participated in the execution and analysis of the study. All authors are aware of and agree to the content of the manuscript, and all authors have approved the final version submitted and their being listed as an author on the manuscript.
Disclaimer
The contents of this manuscript have not been copyrighted or published earlier. There are no directly related manuscripts or abstracts, published or unpublished, by one or more authors of this manuscript. The contents of this manuscript are not now under consideration for publication elsewhere. Neither the submitted manuscript nor any similar manuscript, in whole or in part, will be copyrighted, submitted, or published elsewhere while the journal is under consideration.
Rights and permissions
About this article
Cite this article
Ortega, F., Vazquez-Martin, A., Moreno-Navarrete, JM. et al. Thyroid hormone responsive Spot 14 increases during differentiation of human adipocytes and its expression is down-regulated in obese subjects. Int J Obes 34, 487–499 (2010). https://doi.org/10.1038/ijo.2009.263
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.263
Keywords
This article is cited by
-
Genetic regulation and variation of expression of miRNA and mRNA transcripts in fetal muscle tissue in the context of sex, dam and variable fetal weight
Biology of Sex Differences (2022)
-
Insulin-inducible THRSP maintains mitochondrial function and regulates sphingolipid metabolism in human adipocytes
Molecular Medicine (2022)
-
Elevated serum S14 levels are associated with more severe liver steatosis by ultrasonography
Scientific Reports (2021)
-
Inflammation and insulin resistance exert dual effects on adipose tissue tumor protein 53 expression
International Journal of Obesity (2014)