Abstract
Objective:
Circadian mechanisms underlie the physiology of mammals as an adaptation to the earth's rotation on its axis. Highly conserved core circadian regulatory proteins (CCRPs) maintain an oscillatory expression profile in the central and peripheral tissues. The CCRP include both a positive and negative arm, as well as downstream transcriptional regulators. Recent studies in murine models have determined that the mRNAs encoding the CCRP are present in multiple adipose tissue depots and exhibit a robust oscillatory expression profile. This study set out to examine the expression of CCRP mRNAs in human subcutaneous adipose tissues.
Design:
Retrospective analysis of total RNA isolated from subcutaneous adipose tissue.
Subjects:
A total of 150 healthy female and male lean (body mass index (BMI) <25), overweight (BMI between 25 and 29.99) or obese (BMI >30) subjects of varied ethnic backgrounds undergoing elective liposuction or surgical procedures.
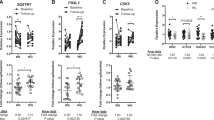
Results:
The expression of the CCRP mRNAs displayed a significant correlation between each other and mRNAs representative of adipogenic biomarkers. Hierarchical cluster analyses of mRNAs isolated from the cohort of female Caucasian subjects (n=116) identified three major clusters based on expression of downstream CCRP mRNAs. The mRNAs encoding D site of albumin promoter-binding protein (DBP), E4 promoter-binding protein 4 (E4BP4), PPARγ coactivator-1β (PGC-1β) and Rev-erbα were negatively correlated with BMI in a lean cluster (n=66), positively correlated with BMI in a younger overweight/obese cluster (n=19), and not significantly correlated with BMI in an older, overweight/obese cluster (n=31).
Conclusions:
These data confirm and extend findings that link the CCRP and circadian mechanisms to the risk of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lowrey PL, Takahashi JS . Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 2004; 5: 407–441.
Hirota T, Fukada Y . Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci 2004; 21: 359–368.
Liu C, Li S, Liu T, Borjigin J, Lin JD . Transcriptional coactivator PGC-1alpha integrates the mammalian Clock and energy metabolism. Nature 2007; 447: 477–481.
Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM . PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA 2007; 104: 5223–5228.
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005; 308: 1043–1045.
Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007; 6: 414–421.
Barnea M, Madar Z, Froy O . High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology 2009; 150: 161–168.
Kaneko K, Yamada T, Tsukita S, Takahashi K, Ishigaki Y, Oka Y et al. Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res 2009; 1263: 58–68.
Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2004; 2: e377.
Scott EM, Carter AM, Grant PJ . Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008; 32: 658–662.
Prasai MJ, George JT, Scott EM . Molecular clocks, type 2 diabetes and cardiovascular disease. Diab Vasc Dis Res 2008; 5: 89–95.
Monteleone P, Tortorella A, Docimo L, Maldonato MN, Canestrelli B, De Luca L et al. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: association with higher body mass index. Neurosci Lett 2008; 435: 30–33.
Sookoian S, Gemma C, Gianotti TF, Burgueno A, Castano G, Pirola CJ . Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr 2008; 87: 1606–1615.
King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP et al. Positional cloning of the mouse circadian clock gene. Cell 1997; 89: 641–653.
Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD et al. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell 1997; 89: 655–667.
Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 2003; 301: 379–383.
Franken P, Lopez-Molina L, Marcacci L, Schibler U, Tafti M . The transcription factor DBP affects circadian sleep consolidation and rhythmic EEG activity. J Neurosci 2000; 20: 617–625.
Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F . Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 2005; 28: 395–409.
Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 1999; 400: 169–173.
Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, Katoh M et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep 2001; 2: 342–346.
Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol 2007; 17: 613–618.
Viola AU, James LM, Archer SN, Dijk DJ . PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol 2008; 295: H2156–H2163.
Mishima K, Tozawa T, Satoh K, Saitoh H, Mishima Y . The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet B Neuropsychiatr Genet 2005; 133B: 101–104.
Knutson KL, Spiegel K, Penev P, Van Cauter E . The metabolic consequences of sleep deprivation. Sleep Med Rev 2007; 11: 163–178.
Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E . Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol 2005; 99: 2008–2019.
Spiegel K, Tasali E, Penev P, Van Cauter E . Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141: 846–850.
Van Cauter EV, Polonsky KS, Blackman JD, Roland D, Sturis J, Byrne MM et al. Abnormal temporal patterns of glucose tolerance in obesity: relationship to sleep-related growth hormone secretion and circadian cortisol rhythmicity. J Clin Endocrinol Metab 1994; 79: 1797–1805.
Van Cauter E, Polonsky KS, Scheen AJ . Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 1997; 18: 716–738.
Chaput JP, Despres JP, Bouchard C, Tremblay A . Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007; 15: 253–261.
Chaput JP, Despres JP, Bouchard C, Tremblay A . The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 2008; 31: 517–523.
Moreno CR, Louzada FM, Teixeira LR, Borges F, Lorenzi-Filho G . Short sleep is associated with obesity among truck drivers. Chronobiol Int 2006; 23: 1295–1303.
Gunderson EP, Rifas-Shiman SL, Oken E, Rich-Edwards JW, Kleinman KP, Taveras EM et al. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol 2008; 167: 178–187.
Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW . Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med 2008; 162: 305–311.
Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008; 32: 1825–1834.
Patel SR, Hu FB . Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008; 16: 643–653.
Wilsbacher LD, Yamazaki S, Herzog ED, Song EJ, Radcliffe LA, Abe M et al. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice in vivo. Proc Natl Acad Sci USA 2002; 99: 489–494.
Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 2004; 101: 5339–5346.
Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T et al. Rhythmic mRNA expression of Clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 2005; 146: 5631–5636 (E-pub 2005 Sep).
Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 2006; 55: 962–970.
Bray MS, Young ME . Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev 2007; 8: 169–181.
Gomez-Abellan P, Hernandez-Morante JJ, Lujan JA, Madrid JA, Garaulet M . Clock genes are implicated in the human metabolic syndrome. Int J Obes (Lond) 2008; 32: 121–128.
Wu X, Zvonic S, Floyd ZE, Kilroy G, Goh BC, Hernandez TL et al. Induction of circadian gene expression in human subcutaneous adipose-derived stem cells. Obesity (Silver Spring) 2007; 15: 2560–2570.
Hernandez-Morante JJ, Gomez-Santos C, Milagro F, Campion J, Martinez JA, Zamora S et al. Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int J Obes (Lond) 2009; 33: 473–480 (E-pub 2009 Feb).
Wu X, Yu G, Parks H, Hebert T, Goh BC, Dietrich MA et al. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone 2008; 42: 861–870.
Everitt BS, Landau S, Leese M . Cluster Analysis. 4th edn Hodder Arnold Publication, 2001.
Zvonic SFZ, Mynatt RL, Gimble JM . Circadian rhythms and the regulation of metabolic tissue function and energy homeostasis. Obesity(Silver Spring) 2007; 15: 539.
Duez H, Staels B . Rev-erb alpha gives a time cue to metabolism. FEBS Lett 2008; 582: 19–25.
Duez H, Staels B . The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diab Vasc Dis Res 2008; 5: 82–88.
Ozkurt IC, Pirih FQ, Tetradis S . Parathyroid hormone induces E4bp4 messenger ribonucleic acid expression primarily through cyclic adenosine 3′,5′-monophosphate signaling in osteoblasts. Endocrinology 2004; 145: 3696–3703.
Ozkurt IC, Tetradis S . Parathyroid hormone-induced E4BP4/NFIL3 down-regulates transcription in osteoblasts. J Biol Chem 2003; 278: 26803–26809.
Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME . Playing with bone and fat. J Cell Biochem 2006; 98: 251–266.
Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA et al. Rev-erb alpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007; 318: 1786–1789.
Burris TP . Nuclear hormone receptors for heme: REV-ERB{alpha} and REV-ERB{beta} are Ligand-Regulated Components of the Mammalian Clock. Mol Endocrinol 2007; 22:1509–1520.
Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol 2007; 14: 1207–1213.
Rogers PM, Ying L, Burris TP . Relationship between circadian oscillations of Rev-erbalpha expression and intracellular levels of its ligand, heme. Biochem Biophys Res Commun 2008; 368: 955–958.
Chen JJ, London IM . Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell 1981; 26: 117–122.
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98: 115–124.
Acknowledgements
We thank Elizabeth Clubb, MD, Thomas Guillot, MD, James Wade, MD, their office staff and patients for donation of lipoaspirate tissues; Laura Dallam for administrative assistance. This work was partially supported by a CNRU Center Grant # 1P30 DK072476 entitled ‘Nutritional Programming: Environmental and Molecular Interactions’ sponsored by NIDDK (GY, SRS and JMG) and the Pennington Biomedical Research Foundation (XW and JMG).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, X., Xie, H., Yu, G. et al. Expression profile of mRNAs encoding core circadian regulatory proteins in human subcutaneous adipose tissue: correlation with age and body mass index. Int J Obes 33, 971–977 (2009). https://doi.org/10.1038/ijo.2009.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.137
Keywords
This article is cited by
-
Prospective influences of circadian clocks in adipose tissue and metabolism
Nature Reviews Endocrinology (2011)