Abstract
Endothelial damage is repaired by endothelial progenitor cells (EPCs), which are pivotal in preventing cardiovascular diseases and prolonging lifespan. The WHO Cardiovascular Diseases and Alimentary Comparison Study demonstrated that dietary taurine and magnesium (Mg) intake suppresses cardiovascular diseases. We herein evaluate the effects of taurine and Mg supplementation on EPC function and oxidative stress in healthy men and spontaneously hypertensive rats (SHRs). Healthy men received taurine (3 g per day) or Mg (340 mg per day) for 2 weeks. SHRs and Wistar-Kyoto (WKY) rats were housed with high-salt drinking water (1% NaCl). The SHRs received 3% taurine solution and/or a high-Mg (600 mg per 100 g) diet for 4 weeks. Their peripheral blood mononuclear cells were separated to quantify EPC colony formation. Oxidative stress markers in their peripheral blood were evaluated using a free radical analytical system and a thiobarbituric acid reactive substance (TBARS) assay. Taurine and Mg supplementation significantly increased EPC colony numbers and significantly decreased free radical levels and TBARS scores in healthy men. Taurine and Mg supplementation significantly increased EPC colony numbers and significantly decreased TBARS scores and free radical levels in SHRs. Nicotinamide adenine dinucleotide phosphate oxidase component mRNA expression was significantly higher in the renal cortex of salt-loaded SHRs than in WKY rats, in which it was suppressed by taurine and Mg supplementation. Taurine and Mg supplementation increased EPC colony formation in healthy men and improved impaired EPC function in SHRs through antioxidation, indicating that the dietary intake of taurine and Mg may prolong lifespan by preventing the progression of cardiovascular diseases.
Similar content being viewed by others
Introduction
Endothelial progenitor cells (EPCs) induce angiogenesis, neovascularization and endothelial repair by homing in on sites of endothelial damage.1 Hill et al.2 demonstrated a reduction of EPC colony formation in healthy individuals with risk factors for cardiovascular disease. EPC numbers have also been shown to be reduced in patients with other disorders who are at higher risk for cardiovascular diseases.3 Hence, it is more likely that the pathway controlling EPC release is downregulated by the presence of risk factors rather than by the consumption of EPCs. Accumulating evidence indicates that reactive oxygen species have major roles in the initiation and progression of the cardiovascular dysfunction associated with diseases such as hyperlipidemia, diabetes mellitus, hypertension and ischemic heart disease.4 We have demonstrated that the formation and function of EPCs is impaired in hypertensive rats with increases in oxidative stress and that treatment with angiotensin II receptor blockers (ARBs)5, 6, 7 and a hydroxymethylglutaryl-CoA reductase inhibitor statin8 apparently improved the impaired function of EPCs by suppressing oxidative stress in spontaneously hypertensive rats (SHR). We also recently demonstrated that basal EPC function was inversely correlated with blood pressure and impaired in patients with essential hypertension, and that treatment with an ARB significantly improved their impaired EPC function.9 Thus, the administration of ARBs and statins, which have an antioxidative effect, is an effective therapeutic strategy that promotes the repair of cardiovascular damage by enhancing the repair of endothelial damage by EPCs.
On the other hand, the long-term ingestion of foods that contain high levels of antioxidants also acts to protect against oxidative stress. Vitamin E and C have antioxidant and membrane stabilizing functions.10 β-carotene prevents the oxidative modification of low-density lipoprotein cholesterol.11 Polyphenols may also stimulate endogenous antioxidant enzymes and inhibit xanthine and nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidases.12 Taurine, which is another potent antioxidant that is found in food, has been reported to protect against endothelial damage.13 Because nutrition is a fundamental part of life, consuming these antioxidative foods may be more important than medicines for repairing cardiovascular damage, preventing cardiovascular disease and prolonging the lifespan.
Yamori et al.14 performed the WHO Cardiovascular Diseases and Alimentary Comparison Study to investigate the close relationship between cardiovascular diseases and dietary customs. A regional correlation analysis revealed a significant inverse correlation between the amount of taurine excreted in 24-hour urine and the mortality rates due to coronary heart disease. Moreover, the mortality rates were found to be significantly lower in the regions where large amounts of taurine are consumed, such as Japan. These investigations suggested that the risk of cardiovascular disease was heterogeneous and that it could be greatly influenced by environmental factors, such as dietary customs. The cardiovascular risk factors of individuals in whom the excretion of both taurine and magnesium (Mg) was detected in 24-hour urine were proven to be significantly lower. As taurine and Mg are biomarkers for the consumption of seafood, vegetables, soy, nuts, milk and so on, the consumption of these food sources could be recommended to prevent cardiovascular disease.14
Taurine- and Mg-rich foods are considered to protect against cardiovascular disease and to exert anti-aging effects. It is possible that the dietary intake of taurine and Mg enhance the function of EPCs in the repair of cardiovascular damage. In the current study, we elucidated the effects of taurine and Mg supplementation on oxidative stress and EPC function in humans and SHRs, which are an animal model of hypertension and oxidative stress.
Subjects and methods
The design of the clinical study
This randomized, double-blind, placebo-controlled study was approved by the Ethics Committee of Nihon University School of Medicine (Authorization No. 2-1-42). The study population included 125 healthy men (age: 18–25 years). We obtained written informed consent from all the participants. Table 1 shows the characteristics of the healthy participants. All of the participants were men. Their body mass index, blood pressure and plasma glucose levels and lipid profiles were within the normal ranges and did not differ among the placebo, taurine and Mg intake groups. The smoking rates of the three groups were 18–24%. All of the participants were completely restricted from consuming fish and shellfish for 1 month before and after the clinical studies. Peripheral blood samples were taken to determine the baseline high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, triglyceride and fasting plasma glucose levels of all the subjects. Serum cholesterol and triglyceride levels were measured by enzymatic methods (Nescauto VL TC and Nescauto VL TG; Nippon Shoji, Osaka, Japan). Peripheral blood mononuclear cells (MNCs) were separated to quantify EPC colony formation. Oxidative stress in the peripheral blood was evaluated by diacron-reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) using a free radical analytical system 4, and a thiobarbituric acid reactive substance (TBARS) assay. As a nutritional intervention study, the participants received 3 g per day as previous report15 of taurine (1 g per capsule 3 times per day; Vegetarian Caps, Allergy Research Group, Alameda, CA, USA) or 340 mg per day of Mg (170 mg per capsule twice per day; Allergy Research Group) for 2 weeks as a double blind test. Placebo capsules, containing 500 mg of lactose hydrate per capsule (Maruish Pharm, Osaka, Japan), were identical in appearance to placebo capsules containing white flour. All of the capsules were packaged in resealable storage bags. Randomization was conducted by the lead investigator who did not have contact with participants or conduct the data entry or blood analyzes. Twenty four-hour urine samples were collected using an aliquot cup.16 All collected urine samples were frozen at −20 °C until analysis. Taurine was measured using an amino acid analyzer (Hitachi 835, Ibaraki, Japan).17 Mg was measured using a colorimetric method.
The experimental design of the animal study
Our investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, 1996). The research protocols involving the use of living animals were assessed and approved by the ethics committee of the Nihon University School of Medicine (AP12M027).
Eleven-week-old male SHR/Izm and Wistar-Kyoto/Izm (WKY/Izm) rats, which were supplied by the Disease Model Cooperative Research Association (DMCRA, Kyoto, Japan), through Japan SLC, Inc. (Hamamatsu, Japan). All rats were housed in an animal facility with a 12-hour light–dark cycle and ad libitum access to standard chow and water. Systolic blood pressure was measured by the tail-cuff method at the start of the 12 weeks of age and at 1-week intervals thereafter. At 12 weeks of age, 3 SHR/Izm and WKY/Izm rats were housed per cage. The temperature, humidity and light were controlled and the animals had unrestricted access to water. In the control group, WKY rats (n=6) and SHRs (n=6) received a low-Mg (60 mg per 100 g) diet (Oriental Yeast, Tokyo, Japan) with high-salt drinking water (1% NaCl) for 4 weeks. In the Taurine group, SHRs (n=6) received 3% taurine solution in high-salt drinking water (1% NaCl) for 4 weeks. In the Mg group, SHRs (n=6) received a high-Mg (360 mg per 100 g) diet (Oriental Yeast) with 1% NaCl for 4 weeks. In the Taurine+Mg group, SHRs (n=6) received 3% taurine solution and a high-Mg diet with 1% NaCl for 4 weeks. The rats were anesthetized with the inhalation of 5% isoflurane after 4 weeks of treatment. Ten milliliters of peripheral blood was sampled and the kidneys were flushed with ice-cold phosphate-buffered saline and immediately removed.
EPC colony forming assay
A modified EPC colony forming assay was performed as described by Hill et al.2 In brief, 20 ml of heparinized peripheral blood was collected once from normotensive controls. The blood samples from healthy men were taken before and after 4 weeks of treatment at Nihon University Hospital. In the animal experiments, the rats were anesthetized with the intraperitoneal administration of sodium pentobarbital (50 mg kg−1) receiving the taurine and/or an Mg diet for 2 weeks and 10 ml of peripheral blood was sampled.
MNCs were separated by centrifugation with Histopaque-1083 density gradient medium (Sigma-Aldrich, St. Louis, MO, USA) and suspended and mixed in 1 ml of EGM-2 medium (Clonetics, San Diego, CA, USA) containing 10% fetal bovine serum, 4 ml l−1 human basic fibroblast growth factor (Sigma-Aldrich), 1 ml l−1 vascular endothelial growth factor (R&D System, Minneapolis, MN, USA), 1 ml l−1 recombinant insulin-like growth factor-1 (Sigma-Aldrich), 1 ml l−1 human epidermal growth factor (Sigma-Aldrich), 1 ml l−1 ascorbic acid, and 1 ml l−1 GA-1000 (BioWhittaker, Walkersville, MD, USA). Twenty-four-well plates (Falcon, Sa Jose, CA, USA) were pre-coated with rat vitronectin (0.1 μg cm−2) plus 0.5% gelatin overnight at 37 °C. MNCs were inoculated into 6-well plates (106 cells per well) and cultured for 24 h. Non-adherent MNCs were reinoculated into the vitronectin-coated 24-well plates (106 cells per well) and cultured in a CO2 incubator at 37 °C for 7 days. The average number of colonies was calculated manually from a minimum of four wells under a light microscope by an observer who was unaware of the experimental design.
TBARS assay
The TBARS concentration in MNCs was measured using a commercial kit (Oxi-Tek TBARS Assay Kit; Zeptometrix, Buffalo, NY, USA).18 In brief, MNCs (106 per 100 μl) were mixed with 100 μl of sodium dodecyl sulfonate solution. TBA/buffer reagent was prepared by mixing 0.5 g of TBA with 50 ml of acetic acid and 50 ml of NaOH. TBA/buffer reagent (2.5 ml) was added to 200 μl of sample/sodium dodecyl sulfonate mixture and incubated at 95 °C in capped tubes for 60 min. The samples were cooled to room temperature in an ice bath for 10 min and centrifuged at 300 × g for 15 min. The supernatants were removed, and the fluorescence intensity was measured in semi-micro cuvettes using a fluorometer (Bio-Rad, Hercules, CA, USA). The TBARS concentration (measured as pmol per 106 cells) was interpolated from the standard curve of malondialdehyde at concentrations of 0–200 pmol l−1.
The analysis of oxidative stress using a free radical analytical system 4
The level of oxidative stress in the peripheral blood was measured using a free radical analytical system 4 (WISMERLL, Tokyo Japan) as described previously.19
Urinary sampling
Twenty-four-hour urine was collected using an aliquot cup as described previously.16 Urinary taurine excretion was measured using an amino acid analyzer (Hitachi 835).
The real-time PCR of NAD(P)H oxidase components
Total RNA was isolated from rat kidney with ISOGEN (Nippon Gene, Toyama, Japan) according to the manufacturer's instructions. Aliquots of total RNA were reverse transcribed into single-stranded cDNA by incubation with avian myeloblastoma virus reverse transcriptase (Takara Biochemicals, Shiga, Japan). The tissue cDNA levels were measured by a real-time PCR with an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. All of the probes and primers were Assays-on-Demand Gene Expression Products (p22phox, Rn00577357_ml; p47phox, Rn00586945_ml; gp91phox, Rn00576710_ml and TaqMan Rodent glyceraldehyde 3-phosphate dehydrogenase control reagents, Applied Biosystems). Each reaction mixture consisted of 12.5 μl of TaqMan Universal PCR Master Mix (Applied Biosystems), 50 ng of cDNA template and 1.25 ml of the specific probe/primer mixed in a total volume of 25 μl. The amplification conditions were as follows: 50 °C for 2 min and 95 °C for 10 min followed by 60 cycles of denaturation (95 °C for 15 s) and combined annealing/extension (60 °C for 1 min). The threshold cycle (Ct) was subsequently determined. The relative quantification of marker gene mRNA expression was calculated with the comparative Ct method. The relative quantification value of the target, normalized to an endogenous control (GAPDH gene) and relative to a calibrator, was expressed as 2-ddCt (fold difference).
Statistics
The data were analyzed by Student's t-test or Student's unpaired t-test as appropriate. The results are expressed as the mean±s.e.m. A multiple regression analysis, which included the systolic blood pressure, diastolic blood pressure and EPC colony formation number, was conducted using the stepwise method with other independent variables using the StatView software program (version 5.0, SAS Institute, Cary, NC, USA).
Results
The effects of taurine and Mg supplementation on EPC colony formation in healthy men
Typical EPC colony formation numbers are shown in Figures 1a and b. The basal levels of urinary taurine and Mg were not correlated with basal EPC colony formation in healthy men (Figures 1c and d). We confirmed that there were significant (P<0.01) increases in the urinary excretion of taurine after 2 weeks of taurine (3 g per day) supplementation (Figure 1f). Two weeks of taurine (3 g per day) and Mg (340 mg per day) supplementation significantly (P<0.01) increased EPC colony formation in healthy men (Figure 2). EPC colony formation did not increase in the placebo group.
Representative micrographs of human endothelial progenitor cell (EPC) colony formation. After 7 days of culture, nonadherent cells were removed, and attached cells formed distinct colonies (a: × 200, b: × 40). The relationship of the basal levels of urinary taurine and magnesium (Mg) excretion with basal EPC colony formation in healthy men. One-hundred twenty-five healthy men (age: 18–25 years) participated in this study. They received taurine (3 g per day) (c) or Mg (340 mg per day) (d) for 2 weeks. The placebo group (e) received capsules containing lactose hydrate (500 mg). (f) Increases in the urinary excretion of taurine after 2 weeks of taurine (3 g per day) supplimentation.
The effects of taurine and magnesium (Mg) supplementation on endothelial progenitor cell (EPC) colony formation in healthy men. Subjects in the placebo group (a, n=42) received capsules lactose hydrate (500 mg). Healthy men in the Taurine and Mg groups received taurine (3 g) (b, n=45) or Mg (340 mg) (c, n=38), respectively, for 2 weeks. The data were expressed as the mean±s.e.m. **P<0.01 vs. baseline (before supplementation).
The effects of taurine and Mg supplementation on oxidative stress in healthy men
Two weeks of Mg (340 mg per day) supplementation significantly (P<0.01) decreased the d-Roms level in healthy men (Figure 3). Two weeks of taurine (3 g per day) supplementation and 2 weeks of Mg supplementation (340 mg per day) significantly increased the BAP score in healthy men (P<0.01 and P<0.05, respectively) (Figure 3). The oxidative stress marker levels were not affected in the placebo group. These data indicate that taurine and Mg supplementation suppressed oxidative stress in healthy men.
The effects of taurine and magnesium (Mg) supplementation on oxidative stress in healthy men. Subjects in the placebo group (a,d, n=42) received capsules lactose hydrate (500 mg). Healthy men in the Taurine and Mg groups received 3 g per day of taurine (b,e, n=45) or 340 mg per day of Mg (c,f n=38), respectively, for 2 weeks. Oxidative stress in the peripheral blood were evaluated based on the diacron-reactive oxygen metabolites (d-ROMs) level, the results of a biological antioxidant potential (BAP) test, which was performed using afree radical analytical system (FRAS 4), and a thiobarbituric acid reactive substance (TBARS) assay. The data are expressed as the mean±s.e.m. *P<0.05 and **P<0.01 vs. baseline (before supplementation).
The effects of taurine and Mg supplementation on body weight, blood pressure and EPC colony formation in SHRs
To investigate the mechanisms underlying the increases in EPC colony formation with taurine and Mg supplementation in healthy men, we examined the effects of taurine and Mg supplementation in SHRs as a hypertensive and oxidative animal model. Taurine and Mg supplementation did not affect changes in body weight or blood pressure in SHRs (Figures 4a and b). The basal EPC colony number was significantly (P<0.01) lower in SHRs than in WKY rats (Figure 5). Taurine (P<0.05) and Mg (P<0.01) supplementation significantly increased the EPC colony numbers in SHRs. In SHRs, the combination of taurine and Mg supplementation resulted in a significant (P<0.01) additional increase in the EPC colony numbers (Figure 5).
The changes in body weight (a) and blood pressure (b) in spontaneously hypertensive rats (SHRs) that received taurine and magnesium (Mg) supplementation. In the Control group, Wistar Kyoto (WKY) rats and SHRs (n=6) received a low-Mg (60 mg per 100 g) diet with high-salt drinking water (1% NaCl) for 4 weeks. In the Taurine group, SHRs (n=6) received a 3% taurine solution in 1% NaCl for 4 weeks. In the Mg group, SHRs (n=6) received a high-Mg (360 mg per 100 g) diet with 1% NaCl for 4 weeks. In the Taurine+Mg group, SHRs (n=6) received 3% taurine solution and a high-Mg diet with 1% NaCl for 4 weeks. Systolic blood pressure was measured by the tail-cuff method at the start of the 12 weeks of age. The data are expressed as mean±s.e.m.
The effects of taurine and magnesium (Mg) supplementation on endothelial progenitor cell (EPC) colony formation in spontaneously hypertensive rats (SHRs). In the Control group, Wistar Kyoto (WKY) rats and SHRs (n=6) received a low-Mg (60 mg per 100 g) diet with high-salt drinking water (1% NaCl) for 4 weeks. In the Taurine group, SHRs (n=6) received a 3% taurine solution in 1% NaCl for 4 weeks. In the Mg group, SHRs (n=6) received a high-Mg (360 mg per 100 g) diet with 1% NaCl for 4 weeks. In the Taurine+Mg group, SHRs (n=6) received 3% taurine solution and a high-Mg diet with 1% NaCl for 4 weeks. Peripheral blood mononuclear cells were separated to quantify EPC colony formation. The data are expressed as the mean±s.e.m. *P<0.05 and **P<0.01 between the indicated columns.
The effects of taurine and Mg supplementation on oxidative stress in SHRs
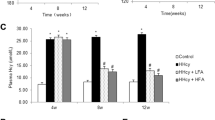
The basal TBARS score in MNCs was significantly (P<0.01) higher in SHRs than in WKY rats, indicating that oxidative stress is stronger in SHRs than in WKY rats (Figure 6a). Taurine (P<0.05), Mg (P<0.01) supplementation and the combination of taurine and Mg supplementation significantly (P<0.01) decreased the TBARS scores of SHRs (Figure 6a). The d-ROMs level in the peripheral blood was higher in SHRs than in WKY rats; this was suppressed to a statistically significant (P<0.01) extent in SHRs that received taurine supplementation and SHRs that received the combination of taurine and Mg supplementation (Figure 6b). The basal BAP level was significantly (P<0.01) lower in SHRs than in WKY rats. Taurine and Mg supplementation tended to increase the BAP level, but not to a statistically significant extent (Figure 6c).
The effects of taurine and magnesium (Mg) supplementation on oxidative stress in spontaneously hypertensive rats (SHRs). In the Control group, Wistar Kyoto (WKY) rats and SHRs (n=6) received a low-Mg (60 mg per 100 g) diet with high-salt drinking water (1% NaCl) for 4 weeks. In the Taurine group, SHRs (n=6) received a 3% taurine solution in 1% NaCl for 4 weeks. In the Mg group, SHRs (n=6) received a high-Mg (360 mg per 100 g) diet with 1% NaCl for 4 weeks. In the Taurine+Mg group, SHRs (n=6) received 3% taurine solution and a high-Mg diet with 1% NaCl for 4 weeks. Peripheral blood mononuclear cells were separated to quantify EPC colony formation. (a) The level of thiobarbituric acid reactive substance (TBARS) in mononuclear cells was measured with a commercial kit. The level of diacron-reactive oxygen metabolites (d-ROMs) (b) and biological antioxidant potential (BAP) (c) in the peripheral blood were measured using a free radical analytical system (FRAS 4). The data are expressed as the mean±s.e.m. *P<0.05 and **P<0.01 between the indicated columns.
The effects of taurine and Mg supplementation on NAD(P)H oxidase in SHRs
To investigate the mechanisms underlying the antioxidative effects of taurine and Mg supplementation, we measured the mRNA expression of NAD(P)H oxidase components after taurine and Mg supplementation in the kidney of SHRs. p22-phox mRNA was significantly (P<0.05) more abundant in the SHR kidney than in the WKY rat kidney. gp91-phox mRNA was significantly (P<0.05) less abundant in the kidney of SHRs that received Mg supplementation than in SHRs that did not receive Mg supplementation (Figure 7a). p22-phox mRNA was significantly (P<0.05) less abundant in the of SHRs that received combined taurine and Mg supplementation than in SHRs that did not receive supplementation (Figure 7b). p47-phox mRNA was significantly (P<0.05) less abundant in the kidney from SHRs that received Mg supplementation than in SHRs that did not receive supplementation (Figure 7c).
The effects of taurine and magnesium (Mg) supplementation on nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase in spontaneously hypertensive rats (SHRs). In the Control group, Wistar Kyoto (WKY) rats and SHRs (n=6) received a low-Mg (60 mg per 100 g) diet with high-salt drinking water (1% NaCl) for 4 weeks. In the Taurine group, SHRs (n=6) received a 3% taurine solution in 1% NaCl for 4 weeks. In the Mg group, SHRs (n=6) received a high-Mg (360 mg per 100 g) diet with 1% NaCl for 4 weeks. In the Taurine+Mg group, SHRs (n=6) received 3% taurine solution and a high-Mg diet with 1% NaCl for 4 weeks. The expression of mRNA of NAD(P)H oxidase components gp91phox (a), p22phox (b), and p47phox (c) in the renal cortex were determined by a real-time reverse transcription PCR. The data are expressed as the mean±s.e.m. *P<0.05 and **P<0.01 between the indicated columns.
Discussion
In the present study, 2 weeks of taurine and Mg supplementation significantly increased the EPC colony numbers in healthy men. It is, therefore, considered that dietary customs that involve the consumption of foods containing taurine and Mg could act to protect against cardiovascular damage by improving endothelial function. The EPC colony-formation number, which reflects the vascular repair function of EPCs, provides a better measurement of the number of EPCs in peripheral blood than the CD34 marker level. With regard to the mechanisms underlying the increase in EPC colony formation that accompanies the dietary consumption of taurine and Mg, 2 weeks of Mg supplementation significantly decreased the level of d-Roms, and taurine and Mg supplementation significantly increased the BAP score in healthy men, indicating that taurine and Mg supplementation suppressed oxidative stress, even in healthy men with lower levels of oxidative stress in comparison to patients with hypertension and diabetes mellitus. We recently demonstrated that basal EPC colony numbers were markedly lower in patients with hypertension than in healthy controls.9 Taurine has been reported to exert antioxidant effects and Ang II antagonism in tissue to improve cardiovascular damage.20 Thus, taurine and Mg supplementation increased the basal EPC colony numbers. This indicates that taurine and Mg supplementation, which can be provided via dietary consumption, is a feasible means of maintaining EPC function and thereby preventing cardiovascular diseases in humans.
We have investigated the formation and function of EPCs in salt-loaded SHRs instead of patients with essential hypertension. Treatment with ARBs,5, 6, 7 an antioxidative β-blocker,21 and a statin8 improved the functional impairment of EPCs in SHRs through the antioxidative effects of these agents. It has been established that the pathogenesis of hypertension and the associated cardiovascular organ damages are associated with increases in Ang II tissue levels.22 Ang II elevation directly induces hypertension and cardiovascular damage by increasing the production of growth factors and oxidative stress. Thus, oxidative stress has been implicated in the pathogenesis of Ang II-related hypertension.23 We found that EPCs themselves express renin–angiotensin system components such as renin, cathepsin D, chymase, Ang II type 1 receptors and Ang II type 2 receptors.6 We have demonstrated that the tissue levels of Ang II are markedly higher in mesenchymal tissues (that is, artery and kidney tissue) in SHRs than in normotensive WKY rats.22, 24 Thus, the presence of Ang II receptors and enzymes in EPCs, therefore, suggests that a paracrine mechanism may be involved in the local and circulating renin–angiotensin system-mediated regulation of oxidative stress for EPC colony formation in hypertension.
Ang II, which acts through the Ang II type I receptor, stimulates NAD(P)H oxidase, induces oxidative stress and impairs endothelial cell function.25 In the present study, we showed that the mRNA expression of NAD(P)H oxidase components (gp91-phox, p22-phox and p47-phox) was increased in the renal cortex of salt-loaded SHRs. Radical oxygen species are produced in the cardiovascular system by various types of cells, including endothelial cells, vascular smooth muscle cells, adventitial fibroblasts and macrophages, primarily through the production of superoxide radicals (O2−). In the cardiovascular system, O2− is produced, in a large part, by the enzyme NAD(P)H oxidase.26 Oxidative stress is increased in salt-loaded SHRs by this mechanism. The lifespan of stem and progenitor cells, including EPCs, has been reported to be shortened by oxidative stress.27 The redox balance changes as cells mature, suggesting that antioxidants may have major roles in preserving the characteristics of stem cells and progenitor cells.
In the present study, the basal TBARS score was significantly higher in SHRs than in WKY rats, indicating that oxidative stress is stronger in SHRs than in WKY rats. The supplementation of taurine Mg and the combination of taurine and Mg significantly decreased the TBARS score in SHRs. The d-Roms level, which was higher in SHRs than in WKY rats, was significantly suppressed by taurine supplementation and by the combination of taurine and Mg supplementation in SHRs. The basal level of BAP was significantly lower in SHRs than in WKY rats. It is, therefore, hypothesized that taurine and Mg supplementation enhance EPC function through the suppression of oxidative stress in salt-loaded SHRs. We investigated the expression of the NAD(P)H oxidase components in the kidney of WKY rats and SHRs with that received taurine and Mg supplementation. In the present study, taurine and Mg supplementation suppressed the expression of the NAD(P)H oxidase components in the kidney of SHRs, suggesting that the antioxidative effects of taurine and Mg supplementation are associated with the inhibition of NAD(P)H oxidase components.
Aging, which reflects the inevitable and progressive degeneration of biological functions due to cumulative metabolic damage, occurs in all living organisms. Oxidative stresses shorten the lifespans of stem cells and progenitor cells, which prevents them from repairing tissue and organ damage, leading to an accumulation of damage and a shortened lifespan. The lifespans of progenitor cells, including EPCs, which are regulated by oxidative mechanisms, influence the length of human life. Antioxidative medicines such as ARBs and statins improve EPC function. Because nutrition is a fundamental part of life, the consumption of antioxidative foods may be more important than the administration of supplements and medicines for the repair of cardiovascular damage. Thus, the dietary consumption of foods that contain taurine and Mg are likely to prolong the lifespan by increasing the number of EPCs.
In conclusion, taurine and Mg supplementation led to increased EPC colony formation in healthy men and improved impaired EPC function in SHRs via antioxidative effects. These findings indicate that the dietary intake of taurine and Mg may prolong lifespan by facilitating the repair of impaired endothelial functions and thereby preventing the progression of cardiovascular diseases.
Limitations
In this study, we did not examine the supplementation of taurine and Mg on the EPC function in patients with hypertension, as almost patients with hypertension in hospital were already treated with antioxidative medicines including ARB, ACE inhibitors and statins that could not be discontinued by ethics reason. Instead of the examination of supplementation of taurine and Mg in patients with hypertension, we employed SHR to clarify the mechanisms of improving effects of the supplementation of taurine and Mg on the EPC function, did not employ WKY rats as we already confirmed improving effects on the supplementation of taurine and Mg on the EPC function in healthy men.
References
Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP . Evidence for circulating bone marrow-derived endothelial cells. Blood 1998; 92: 362–367.
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T . Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348: 593–600.
Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D, Weigel G, Schwarzinger I, Wolozcszuk W, Steiner G, Smolen JS . Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation 2005; 111: 204–211.
Yao EH, Yi Yu, Fukuda N . Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol 2006; 7: 101–108.
Yao EH, Fukuda N, Matsumoto T, Katakawa M, Yamamoto C, Tsunemi A, Suzuki R, Ueno T, Matsumoto K . Losartan improves the impaired function of endothelial progenitor cells in hypertension via an antioxidant effect. Hypertens Res 2007; 30: 1119–1128.
Yu Y, Fukuda N, Yao EH, Matsumoto T, Kobayashi N, Suzuki R, Tahira Y, Ueno T, Matsumoto K . Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension. Am J Hypertens 2008; 21: 72–77.
Yoshida Y, Fukuda N, Maeshima A, Yamamoto C, Matsumoto T, Ueno T, Nojima Y, Matsumoto K, Soma M . Treatment with valsartan stimulates endothelial progenitor cells and renal label-retaining cells in hypertensive rats. J Hypertens 2011; 29: 91–101.
Matsumura M, Fukuda N, Kobayashi N, Umezawa H, Takasaka A, Matsumoto T, Yao E-H, Ueno U, Negishi N . Effects of atorvastatin on angiogenesis in hindlimb ischemia and endothelial progenitor cell formation in rats. J Atheroscler Thromb 2009; 16: 319–326.
Suzuki R, Fukuda N, Katakawa M, Tsunemi A, Tahira Y, Matsumoto T, Ueno T, Soma M . Effects of an angiotensin II receptor blocker on the impaired function of endothelial progenitor cells in patients with essential hypertension. Am J Hypertens 2014; 27: 695–701.
Hennig B, Alvarado A . Nutrition and endothelial cell integrity: implications in atherosclerosis. Prog Food Nutr Sci 1993; 17: 119–157.
Jialal I, Norkus EP, Cristol L, Grundy SM . β-Carotene inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta 1991; 1086: 134–138.
Ulrich-Merzenich G, Zeitler H, Vetter H, Bhonde RR . Protective effects of taurine on endothelial cells impaired by high glucose and oxidized low density lipoproteins. Eur J Nutr 2007; 46: 431–438.
Yu X, Chen K, Wei N, Zhang Q, Liu J, Mi M . Dietary taurine reduces retinal damage produced by photochemical stress via antioxidant and anti-apoptotic mechanisms in Sprague-Dawley rats. Br J Nutr 2007; 98: 711–719.
Yamori Y, Liu L, Mizushima S, Ikeda K, Nara Y, Simpson FO . Male cardiovascular mortality and dietary markers in 25 population samples of 16 countries. J Hypertens 2006; 24: 1499–1505.
Hu TH, Lin CL, Huang YW, Liu PE, Hwang DF . Dietary amino acid taurine ameliorates liver injury in chronic hepatitis patients. Amino Acids 2008; 35: 469–473.
Yamori Y, Nara Y, Kihara M, Mano M, Horie R . Simple method for sampling consecutive 24-hour urine for epidemiological and clinical studies. Clin Exp Hypertens A 1984; 6: 1161–1167.
Yamori Y, Liu L, Mori M, Sagara M, Murakami S, Nara Y, Mizushima S . Taurine as the nutritional factor for the longevity of the Japanese revealed by a world-wide epidemiological survey. Adv Exp Med Biol 2009; 643: 13–25.
Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS . Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 2003; 14: 2775–2782.
Kanaokaa Y, Inagaki E, Hamanaka S, Masaki H, Tanemotob K . Analysis of reactive oxygen metabolites (ROMs) after cardiovascular surgery as a marker of oxidative stress. Acta Med Okayama 2010; 64: 323–330.
Xu YJ, Arneja AS, Tappia PS, Dhalla NS . The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol 2008; 13: 57–65.
Yao EH, Fukuda N, Matsumoto T, Yamamoto C, Suzuki R, Ueno T, Kobayashi N, Matsumoto K . Effects of the antioxidative β-blocker celiprolol on endothelial progenitor cells and oxidation in spontaneously hypertensive rats. Am J Hypertens 2008; 21: 1062–1068.
Fukuda N, Satoh C, Hu WY, Soma M, Kubo A, Kishioka H, Watanabe Y, Izumi Y, Kanmatsuse K . Production of angiotensin II by homogeneous cultures of vascular smooth muscle cells from spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 1999; 19: 1210–1217.
Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS . A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol 2002; 13: 2860–2868.
Hu WY, Fukuda N, Kanmatsuse K . Growth characteristics, angiotensin II-generation, and microarray-determined gene expression in vascular smooth muscle cells from young spontaneously hypertensive rats. J Hypertens 2002; 20: 1323–1333.
Nyby MD, Abedi K, Smutko V, Eslami P, Tuck ML . Vascular angiotensin type 1 receptor expression is associated with vascular dysfunction, oxidative stress and inflammation in fructose-fed rats. Hypertens Res 2007; 30: 451–457.
Taniyama Y, Griendling KK . Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 2003; 42: 1075–1081.
Balin AK, Fisher AJ, Anzelone M, Leong I, Allen RG . Effects of establishing cell cultures and cell culture conditions on the proliferative life span of human fibroblasts isolated from different tissues and donors of different ages. Exp Cell Res 2002; 274: 275–287.
Acknowledgements
The present study was supported by financial grants from the ‘Strategic Research Base Development’ Program for Private Universities for 2008–2012 (S0801033), Nihon University Multidisciplinary Research Grant for 2010–2011 (sou10-045). Program for Private Universities for 2014–2018 (S1411018). The grants were subsidized by MEXT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Katakawa, M., Fukuda, N., Tsunemi, A. et al. Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Hypertens Res 39, 848–856 (2016). https://doi.org/10.1038/hr.2016.86
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.86
Keywords
This article is cited by
-
An Overview of the Risks of Contemporary Energy Drink Consumption and Their Active Ingredients on Cardiovascular Events
Current Cardiovascular Risk Reports (2023)
-
Effects of taurine on vascular tone
Amino Acids (2022)
-
Ameliorative effects of ark clams (Scapharca subcrenata and Tegillarca granosa) on endothelial dysfunction induced by a high-fat diet
Applied Biological Chemistry (2020)
-
Former very preterm infants show alterations in plasma amino acid profiles at a preschool age
Pediatric Research (2017)