Abstract
This meta-analysis aimed to investigate the predictive effect of copeptin as a biomarker for the prognosis of acute ischemic stroke and transient ischemic attack. Electronic databases including PubMed, Medline, EMBASE, Web of Science and Cochrane Central were searched for studies assessing the association of copeptin level on admission with prognosis of acute ischemic stroke and transient ischemic attack. The Newcastle–Ottawa Quality assessment scale for cohort study was used to evaluate quality. A total of 1976 acute ischemic stroke patients from 6 studies were included, and 59% of patients were male. Patients with poor outcomes and nonsurvivors had a higher copeptin level at admission (P<0.0001). Copeptin combined with an admission National Institutes of Health Stroke Scale score significantly improved the discriminatory accuracy of functional outcome and mortality compared with the National Institutes of Health Stroke Scale alone. Elevation in plasma copeptin level carried a higher risk of all-cause mortality (odds ratio=4.16; 95% CI: 2.77–6.25) and poor functional outcome (odds ratio=2.56; 95% CI: 1.97–3.32) after acute ischemic stroke. In addition, copeptin improved the prognostic value of the ABCD2 (age, blood pressure, clinical features of transient ischemic attack, duration of symptoms and presence of diabetes mellitus) score for a recurrent cerebrovascular event in transient ischemic attack. Copeptin seems to be a promising independent biomarker for predicting the functional outcome and all-cause mortality within 3 months or 1 year after acute ischemic stroke, and it could also be a powerful tool for early risk stratification for patients with transient ischemic attack.
Similar content being viewed by others
Introduction
The average annual stroke incidence in seven Chinese cities was 215.6 per 100 000 during the period from 1986 to1990,1 and this was almost twice of that in Japan in 1985.2 Another study from 1986 to 2000 reported that the incidence of stroke increased to 236.6 per 100 000, with the highest incidence of ∼486 per 100 000 in the north and the lowest of 136 per 100 000 in the south.3 Stroke was the second leading cause of mortality in China and one of the leading causes of severe morbidity. Approximately 15–30% of stroke survivors are permanently disabled.4 Ischemic stroke was the most common type of stroke, accounting for 60–80% of all types of stroke.5 Age-standardized incidence rates of total stroke events and ischemic stroke events grew at a speed of 6.7% and 8.7%, respectively.6 The risk of stroke increases after a transient ischemic attack (TIA).7 An early estimate of the severity and prognosis of ischemic stroke and TIA is important in the routine practice of medicine. Therefore, reliable and rapid measurable blood biomarkers to predict the outcome after ischemic stroke and TIA are urgently needed and useful in clinical decision making.
Some studies found that arginine vasopressin was elevated in ischemic stroke patients, and the elevation was correlated with stroke severity.8 However, the clinical application of arginine vasopressin is limited because of a very short half-time (24 min) in vivo,9 and it is still unstable in isolated plasma even stored at −20 °C.10 Furthermore, it is largely attached to platelets11, 12 in plasma. Copeptin, the C-terminal portion of pro-vasopressin, is a neurohypophysis hormone that is a 39-amino acid glycopeptide released in an equimolar ratio to arginine vasopressin. The ex vivo stability of copeptin was shown in serum and plasma for at least 7 days at room temperature and 14 days at 4° C.13 Copeptin can be easily measured using automated assays with interlaboratory coefficient of variation <20% for values >2.25 pmol l−1;14 therefore, it seems to be a sensitive surrogate for arginine vasopressin.12 ABCD2 (age, blood pressure, clinical features of TIA, duration of symptoms and presence of diabetes mellitus)15 and ABCD3-I (ABCD2, dual TIA, imaging)7 are management models based on clinical data and have been used for predicting stroke recurrence after TIA. However, some studies revealed that the ABCD2 score has limited ability to predict stroke risk after TIA.16, 17 In addition, the limited availability of urgent MRI prevents the ABCD3-I score from being widely used.7 Katan et al.18 found that plasma copeptin provides additional prognostic information beyond the ABCD2 clinical risk score for risk stratification in patients with TIA. Some studies18, 19 have demonstrated that copeptin independently predicts functional outcome and mortality in patients with ischemic stroke as well as recurrent cerebrovascular events in patients with TIA. However, most studies are conducted in a single center, and conclusions are not consistent. We performed a meta-analysis to assess the role of copeptin for risk stratification after acute ischemic stroke (AIS) and TIA, aiming to provide powerful evidence for clinical practice.
Methods
This meta-analysis complied with the PRISMA (preferred reporting items for systematic reviews and meta-analysis) guidelines.20
Literature retrieval
Studies were identified by searching electronic databases (PubMed, Medline, EMBASE, Web of Science and Cochrane Central) from inception, reviewing conference proceedings and contacting field experts. The last electronic search was undertaken on 10 December 2015, and search terms included stroke, ischemic stroke, transient ischemic stroke, copeptin, C-terminal pro-vasopressin, mortality, outcome and recurrent cerebrovascular events. Language was limited to English. We also tracked the reference lists of selected articles to identify more studies.
Inclusion and exclusion criteria
Studies that complied with the following criteria were included in this meta-analysis: (1) prospective studies had similar end point criteria and follow-up time of at least 3 months; (2) adult AIS or TIA patients were confirmed according to unified diagnostic criteria; (3) the original data included basic information such as age, proportion of gender, smoking and hypertension, disease history such as diabetes, coronary heart disease, atrial fibrillation and family stroke history, and copeptin levels within 24 h after stroke or TIA onset; and (4) odds ratios (ORs) or hazard ratios (HRs) with their 95% confidence intervals (CIs) of copeptin were reported or could be obtained by other numerical calculation. If repeated reports from the same population were available, only the one with the largest database was included. Studies with poor quality or providing little information were excluded.
Data extraction
Two review authors independently screened the literature, evaluated the quality and extracted information of each study using a data abstraction form. Disagreements were ruled by a third reviewer. Data extraction of AIS patients included study characteristics (the first author’s name, country where the study was performed, publication year, study type, multicenter or not, duration of follow-up, criterion of outcome assessment and number of patients), patient characteristics, baseline copeptin levels the area under receiver operating characteristic curves of copeptin and the National Institutes of Health Stroke Scale (NIHSS), the adjusted OR or HR and 95% CI and possible bias. Data extraction of TIA patients included study characteristics (the first author’s name, country where the study was performed, publication year, study type, multicenter or not, duration of follow-up and number of patients) and ABCD2 score, the adjusted OR or HR and 95% CI and patient characteristics.
Quality assessment
The Newcastle–Ottawa Quality assessment scale for cohort studies21 was used to evaluate study quality because all the included studies were cohorts. Studies were assessed from three aspects: the selection of patients and controls, the comparability of the groups and the evaluation of the outcome. The total score of each study ranged from 0 to 9, and studies with a score of ⩾6 were included.
Statistical analysis
The data of AIS patients were statistically analyzed using Stata10 (College Station, TX, USA), and the OR or HR was a statistic. The heterogeneity test between studies was evaluated using the Cochran’s Q χ2 test with the I2 statistic. An I2 of 25%, 50% and 75% represent mild, moderate and severe inconsistency, respectively. The P-values of >0.1 and I2<50% were taken as indicators of the same scale of outcome, and ORs or HRs were pooled using a fixed effect model. P⩽0.1 and I2⩾50% were taken as indicators of heterogeneity between studies, and ORs or HRs were pooled using a random effects model.22 The source of heterogeneity and sensitivity analysis also needed to be conducted. After a logarithmic transformation of ORs or HRs and 95% CI, pooled ORs or HRs were calculated, and the forest plot was drawn using the metan program. The Z-test was used to assess the pooled ORs or HRs, and P<0.05 was considered significant.
Reporting bias
Evidence of publication bias was assessed statistically and graphically by Begg’s rank correlation test23 and Egger’s regression24 examining a scatter plot of the log of the diagnostic relative risk vs. the inverse of the square root of the effective sample size. A graph shaped symmetrical inverted funnel indicates there is no publication bias.
Results
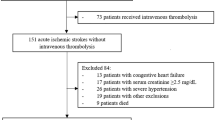
A total of 75 records of AIS and 14 records of TIA were identified through a database search, and all were in English. Of all the records, 80 records were removed, including 54 duplicates, 6 articles with content inconformity and 20 without essential data based on the full-text of articles. Six records of AIS4, 18, 25, 26, 27, 28 (1976 patients) were included in the meta-analysis, and 3 records (646 patients) of TIA7, 19, 29 were analyzed (Figure 1).
Study characteristics and equality evaluation
Study characteristics, baseline characteristics of included stroke patients, NIHSS or ABCD2 score on admission and quality are shown in Table 1. All studies were prospective cohorts published since 2009 with follow-up ranging from 3 months to 1 year. AIS patients from all centers were confirmed in accordance with the unified WHO (World Health Organization) criteria. Functional outcomes of all studies were obtained at 90 days or 1 year according to the modified Rankin Scale score (scores range from 0 to 6, with higher scores indicating increasing severity). A standard definition of TIA was used to confirm patients in cohort studies, whereas functional outcomes of the three included studies were not completely the same.
A total of 1976 AIS patients were included, and 59% of patients were male. In the 6 studies, the median ages of patients were ∼70 years. The distribution of risk factors for common cardiovascular diseases in all AIS patients was as follows: the proportion of smokers was 24.3, 21.5% were diagnosed with atrial fibrillation, 71.6% had a history of hypertension, diabetes patients accounted for 24.4, 24.5% had a family history of stroke and the proportions of patients with hypercholesterolemia and coronary heart disease were 36.7% and 23.0%, respectively. TIA patients were ∼70 years old, and 40–60% of them were male.
Copeptin level
The outcomes of AIS patients were assessed based on the modified Rankin Scale score after follow-up blinded to copeptin levels. As shown in Table 2, all studies found that copeptin levels in patients with poor outcomes were significantly greater than those with good outcomes (all P<0.001). Moreover, nonsurvivors had significantly higher baseline copeptin levels than survivors (all P<0.001). In TIA patients, two studies found that patients with recurrent stroke had significantly higher baseline copeptin levels than no-stroke patients with P-values of 0.02 and 0.088. Katan et al.19 found re-event (TIA or Stroke) patients had significantly higher baseline copeptin levels than no re-event patients (P=0.0016). However, De Marchis et al.7 reported a result with no significance (P=0.63; Table 3).
Predictive value of copeptin, NIHSS and copeptin in combination with NIHSS for AIS outcome and all-cause mortality
NIHSS score (scores range from 0 to 42, and higher scores indicate more serious illness) was performed by one trained medical staff blinded to copeptin levels on admission. In most studies, the area under receiver operating characteristic curves of copeptin was similar to that of the NIHSS score for poor functional outcome and mortality. However, copeptin combined with NIHSS showed a significantly greater discriminatory ability than NIHSS alone (P<0.001) for poor functional outcome and mortality of AIS (Table 4).
Predictive value of copeptin, ABCD2, ABCD3-I and copeptin in combination with ABCD2 or ABCD3-I for stroke
As shown in Table 5, copeptin combined with ABCD2 (P<0.05) or ABCD3-I had a greater discriminatory ability than ABCD2 or ABCD3-I alone for re-stroke after TIA.
Overall effects of copeptin on poor outcome and all-cause mortality of AIS
Five studies4, 18, 25, 26, 28 reported the association of poor functional outcome with log copeptin. A total of 1787 AIS patients were included in this meta-analysis. As shown in Figure 2, the pooled OR of poor outcome was 2.56 (95% CI: 1.97–3.32) for a 10-fold copeptin increase, and the heterogeneity test results were χ2=2.42, P=0.659, I2=0.0%. No evidence of publication bias was found in this meta-analysis according to Begg’s rank correlation Test (P=0.221) and Egger’s regression (P=0.149).
Six studies4, 18, 25, 26, 27, 28 including 1976 AIS patients reported an association between all-cause mortality and log copeptin. As displayed in Figure 3, the pooled OR from four studies18, 25, 27, 28 of all-cause mortality was 4.16 (95% CI: 2.77–6.25) for 10-fold copeptin increase, and the heterogeneity test results were as follows: χ2=0.40, P=0.940, I2=0.0%. Furthermore, publication bias was not found in this meta-analysis (Begg’s test: P=0.308; Egger’s test: P=0.317). Another 2 studies4, 26 reported HRs based on Cox’s proportional hazards regression model, and copeptin level was significantly associated with mortality in AIS patients (HR=2.84; 95% CI: 1.97–4.10; Figure 4). The heterogeneity test results were as follows: χ2=3.60, P=0.058, I2=72.2%.
Effects of copeptin on recurrent events of TIA
We could not estimate the pooled OR value of copeptin because of different study endpoints of TIA patients. De Marchis et al.7 reported that after adjusting for the ABCD2 score, a 10-fold increase in copeptin levels was associated with an OR for stroke of 3.39 (95% CI: 1.28–8.96; P=0.01) after a 3-month follow-up of TIA patients. Katan et al.19 stated in their study that measurement of plasma copeptin in TIA patients provided additional prognostic information beyond the ABCD2 score alone. Purroy et al.29. found that copeptin levels of ⩾13.8 pmol l−1 was an independent predictor of SR (stroke recurrence) at the 7-day follow-up in TIA patients.
Discussion
Our findings suggest that copeptin significantly increased in poor functional outcome patients and nonsurvivors, and a high level of copeptin was associated with an increased risk of all-cause death and poor outcome for AIS patients. Overall, the risks of all-cause death and poor outcome increased 316% and 156%, respectively, with a 10-fold copeptin increase. In addition, copeptin combined with the admission NIHSS score improved the accuracy of outcome and mortality prognosis than the NIHSS score alone. These findings supported the hypothesis that copeptin is a novel and independent biomarker for predicting functional outcome and all-cause mortality within 3 months or 1 year after AIS. Furthermore, copeptin improved the prognostic value of the ABCD2 score for a recurrent cerebrovascular event in TIA patients. The mechanisms of copeptin release after patients sustained AIS have already been proposed. First, copeptin is argued to be an immediate and rapid biomarker of stress response because copeptin is a substantial part of the endocrine stress response.30 Second, cerebral edema after stroke is a main reason for copeptin release that develops very early after onset of cerebral ischemia and may be a major factor in early disability after AIS.31 Therefore, it is not surprising that the body responds to acute and life-threatening diseases, including traumatic brain injury,32 hemorrhagic shock33 or cerebral hemorrhage,34 by a rapid copeptin release. Previous studies have reported the prognostic role of copeptin in various types of cardiovascular disorders. Pozsonyi et al.35 identified copeptin as a predictor of mortality (adjusted HR=2.168, 95% CI: 1.740–2.700 for 1 s.d. increase) among heart failure patients with reduced ejection fraction. A systematic review also suggested that elevation in copeptin carried a similar risk of all-cause mortality to an elevation in troponin (odds ratio 5.84 vs. 6.74, respectively, P=0.67) in acute myocardial infarction36.
Several studies4, 18, 26, 27 have suggested that the lower copeptin group had less risk of mortality by dividing AIS patients into three or four groups according to copeptin levels and comparing their survival time using the product-limit method (Kaplan–Meier curve) after follow-up. Increased copeptin can predict complications after AIS within 3 months, and it is also a dependent indicator of mortality,26 poor functional outcome and early neurologic deterioration of cerebral hemorrhage within 1 year.37 Subgroup analysis for patients undergoing brain MRI demonstrated that copeptin levels were positively correlated with stroke lesion size.4
The results of TIA patients mentioned above could not be pooled because of different study end points. Purroy et al.29 established a cutoff point of 13.8 pmol l−1 for copeptin with a great predictive negative value (97.4%), and they found copeptin levels of ⩾13.8 pmol l−1 (HR=3.9; 95% CI: 1.01–14.4; P=0.039) were independent predictors of stroke recurrence at the 7-day follow-up in TIA patients. De Marchis et al.7, 26 reported that in TIA patients, a 10-fold increase in copeptin level was significantly associated with an adjusted OR of 3.39 (95% CI: 1.28–8.96) for ischemic stroke within 3 months, although they did not find a significant association between copeptin and the composite end point (i.e., ischemic stroke or recurrent TIA) within 3 months.
In the past few years, several predictive models for disability and mortality in stroke patients have been generated, validated and evaluated in terms of accuracy, with values of ∼80% in most models, and many of the models agree on common predictor variables including age,38, 39 severity of stroke38 and admission systolic blood pressure.40 The NIHSS is a credible, effective and comprehensive standardized stroke scale that is widely used in predicting stroke severity and prognosis. However, the use of NIHSS implies special training, and there remains a notable interobserver variability.41 In addition, left hemispheric stroke syndromes show greater NIHSS scores than right hemispheric syndromes.42 Our study endorsed that the prognostic value of copeptin alone was similar to the NHISS score, and copeptin combined with NHISS improved the prognosis value of NHISS score alone, and this could help clinicians in refining the risk stratification for patients with AIS. Copeptin is a blood marker adding additional information beyond the ABCD2 score for the risk of subsequent stroke.7 As shown in Table 5, copeptin combined with ABCD2 significantly improved the prognostic value of ABCD2 scores alone that could be a powerful tool for early risk stratification for patients with TIA.
Bustamante et al. considered that the OR or the C-statistic seemed to not adequately represent whether a biomarker added a predictive value over clinical models.43 One way to quantify the predictive probabilities is the net reclassification improvement (NRI) index that evaluates the net number of patients who are correctly reclassified among various groups at risk, when biomarkers are added to the model.43 Because of the absence of relevant data collection, we were not able to test the NRI of copeptin over clinical information in our meta-analysis. However, a large multicenter and prospective study including 783 AIS patients recently published showed that the combination of copeptin with validated clinical variables, both the NIHSS and age, led to a NRI of 11.8% for poor functional outcome and 37.2% for mortality.26 Copeptin combined with ABCD2 score moved 36.4% of TIA patients who experienced a subsequent stroke to a higher risk category and 18.2% to a lower risk category compared with the ABCD2 score alone.7 The addition of copeptin to a model including age and vascular risk factors corresponded to a relative increase of NRI of 32% (P<0.0001) in TIA patients.44 Therefore, more similar well-designed cohort studies with sufficient sample sizes should be conducted to test the predictive value of copeptin through NRI analysis. This further research is an important step before clinical use.
In general, our results support copeptin’s potential clinical value, although several questions should be answered. First, we used all-cause mortality instead of cause-specific mortality that may have different associations with copeptin because it was arduous to classify death in practice. Second, all included studies lacked repeated and continuous measurements of baseline serum copeptin. In our opinion, future studies of copeptin should address this action to find the best moment for copeptin measurement and its critical values. Even with the above-mentioned limitations, our study also has some strengths that deserve to be mentioned. Our meta-analysis was mainly based on the same categorical studies that were performed with a high level of precision and low heterogeneity. Moreover, all involved studies adequately adjusted for potential confounders, avoiding the likelihood that other confounders influenced the association of copeptin with the outcome and mortality. Therefore, these included prospective studies have provided useful evidence concerning the potential influence of increased copeptin on AIS or TIA patients.
In conclusion, our study suggests that the elevation of plasma copeptin level in stroke patients indicates that they need further evaluation to determine early therapeutic interventions and aggressiveness in care to improve the prognosis. In addition, most current studies focused on the risk of log copeptin, and further trials should determine threshold or normal ranges of copeptin.
References
Cheng XM, Ziegler DK, Lai YH, Li SC, Jiang GX, Du XL, Wang WZ, Wu SP, Bao SG, Bao QJ . Stroke in China, 1986 through 1990. Stroke 1995; 26: 1990–1994.
Fukiyama K, Kimura Y, Wakugami K, Muratani H . Incidence and long-term prognosis of initial stroke and acute myocardial infarction in Okinawa, Japan. Hypertens Res 2000; 23: 127–135.
Yang QD, Niu Q, Zhou YH, Liu YH, Xu HW, Gu WP, Tian FF, Xie YQ, Zhang L, Xia J . Incidence of cerebral hemorrhage in the Changsha community. A prospective study from 1986 to 2000. Cerebrovasc Dis 2004; 17: 303–313.
Zhang JL, Yin CH, Zhang Y, Zhao LB, Fu HJ, Feng JC . Plasma copeptin and long-term outcomes in acute ischemic stroke. Acta Neurol Scand 2013; 128: 372–380.
Zhang GH, He ML . Association between blood pressure variability and risk of stroke (Chinese). Chin J Neuromed 2014; 13: 749–752.
Yong H, Foody J, Linong J, Dong Z, Wang Y, Ma L, Meng HJ, Shiff S, Dayi H . A systematic literature review of risk factors for stroke in China. Cardiol Rev 2013; 21: 77–93.
De Marchis GM, Weck A, Audebert H, Benik S, Foerch C, Buhl D, Schuetz P, Jung S, Seiler M, Morgenthaler NG, Mattle HP, Mueller B, Christ-Crain M, Arnold M, Katan M . Copeptin for the prediction of recurrent cerebrovascular events after transient ischemic attack: results from the CoRisk study. Stroke 2014; 45: 2918–2923.
Barreca T, Gandolfo C, Corsini G, Del Sette M, Cataldi A, Rolandi E, Franceschini R . Evaluation of the secretory pattern of plasma arginine vasopressin in stroke patients. Cerebrovasc Dis 2001; 11: 113–118.
Baumann G, Dingman JF . Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest 1976; 57: 1109–1116.
Robertson GL, Mahr EA, Athar S, Sinha T . Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest 1973; 52: 2340–2352.
Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB . Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 1983; 5 (2 Pt 2): I129–I138.
Gunebakmaz O, Celik A, Inanc MT, Duran M, Karakaya E, Tulmac M, Akpek M, Sarli B, Ergin A, Topsakal R . Copeptin level and copeptin response to percutaneous balloon mitral valvuloplasty in mitral stenosis. Cardiology 2011; 120: 221–226.
Morgenthaler NG, Struck J, Alonso C, Christ-Crain M, Müller B, Bergmann A . Measurement of the very stable vasopressin precursor copeptin as alternative to vasopressin in the clinical routine. Exp Clin Endocrinol Diabetes 2006; 114 (S1): P16_203.
Morgenthaler NG, Struck J, Alonso C, Bergmann A . Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006; 52: 112–119.
Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, Sidney S . Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369: 283–292.
Purroy F, Jimenez Caballero PE, Gorospe A, Torres MJ, Alvarez-Sabin J, Santamarina E, Martinez-Sanchez P, Canovas D, Freijo MM, Egido JA, Giron JM, Ramirez-Moreno JM, Alonso A, Rodriguez-Campello A, Casado I, Delgado-Medeiros R, Marti-Fabregas J, Fuentes B, Silva Y, Quesada H, Cardona P, Morales A, de la Ossa N, Garcia-Pastor A, Arenillas JF, Segura T, Jimenez C, Masjuan J . Prediction of early stroke recurrence in transient ischemic attack patients from the PROMAPA study: a comparison of prognostic risk scores. Cerebrovasc Dis 2012; 33: 182–189.
Hill MD . 2012 - Review: a dichotomized ABCD2 score has limited ability to predict stroke risk less than or equal to 90 days after TIA. ACP J Club 2013; 158: 1–1.
Katan M, Fluri F, Morgenthaler NG, Schuetz P, Zweifel C, Bingisser R, Muller K, Meckel S, Gass A, Kappos L, Steck AJ, Engelter ST, Muller B, Christ-Crain M . Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol 2009; 66: 799–808.
Katan M, Nigro N, Fluri F, Schuetz P, Morgenthaler NG, Jax F, Meckel S, Gass A, Bingisser R, Steck A, Kappos L, Engelter S, Muller B, Christ-Crain M . Stress hormones predict cerebrovascular re-events after transient ischemic attacks. Neurology 2011; 76: 563–566.
Moher D, Liberati A, Tetzlaff J, Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341.
Wells G, Shea B, O'Connell D, Peterson D, Welch V, Losos M, Tugwell P . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Higgins JP, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560.
Begg CB, Mazumdar M . Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101.
Egger M, Smith GD, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Dong X, Tao DB, Wang YX, Cao H, Xu YS, Wang QY . Plasma copeptin levels in Chinese patients with acute ischemic stroke: a preliminary study. Neurol Sci 2013; 34: 1591–1595.
De Marchis GM, Katan M, Weck A, Fluri F, Foerch C, Findling O, Schuetz P, Buhl D, El-Koussy M, Gensicke H, Seiler M, Morgenthaler N, Mattle HP, Mueller B, Christ-Crain M, Arnold M . Copeptin adds prognostic information after ischemic stroke: results from the CoRisk study. Neurology 2013; 80: 1278–1286.
Wang CW, Wang JL, Zhang Y, Li Q, Guo SX, Ji SB . Plasma levels of copeptin predict 1-year mortality in patients with acute ischemic stroke. Neuroreport 2014; 25: 1447–1452.
Tu WJ, Dong X, Zhao SJ, Yang DG, Chen H . Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J. Neuroendocrinol 2013; 25: 771–778.
Purroy F, Suárez-Luis I, Cambray S, Farré J, Benabdelhak I, Mauri-Capdevila G, Sanahuja J, Quílez A, Begué R, Gil MI, Molina-Seguin J, Torreguitart N . The determination of copeptin levels helps management decisions among transient ischaemic attack patients. Acta Neurol Scand 2015; 134: 140–147.
Katan M, Morgenthaler N, Widmer I, Puder JJ, Konig C, Muller B, Christ-Crain M . Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett 2008; 29: 341–346.
Shuaib A, Xu Wang C, Yang T, Noor R . Effects of nonpeptide V(1) vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke 2002; 33: 3033–3037.
Dong X-Q, Yu W-H, Zhang Z-Y, Du Q, Yang D-B, Shen Y-F, Wang H, Zhu Q, Che Z-H, Liu Q-J . Comparison of the performances of copeptin and multiple biomarkers in long-term prognosis of severe traumatic brain injury. Peptides 2014; 60: 13–17.
Morgenthaler NG, Muller B, Struck J, Bergmann A, Redl H, Christ-Crain M . Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock 2007; 28: 219–226.
Zweifel C, Katan M, Schuetz P, Siegemund M, Morgenthaler NG, Merlo A, Mueller B, Christ-Crain M . Copeptin is associated with mortality and outcome in patients with acute intracerebral hemorrhage. BMC Neurol 2010; 10: 34.
Pozsonyi Z, Forhecz Z, Gombos T, Karadi I, Janoskuti L, Prohaszka Z . Copeptin (C-terminal pro arginine-vasopressin) is an independent long-term prognostic marker in heart failure with reduced ejection fraction. Heart Lung Circ 2015; 24: 359–367.
Lipinski MJ, Escarcega RO, D'Ascenzo F, Magalhaes MA, Baker NC, Torguson R, Chen F, Epstein SE, Miro O, Llorens P, Giannitsis E, Lotze U, Lefebvre S, Sebbane M, Cristol JP, Chenevier-Gobeaux C, Meune C, Eggers KM, Charpentier S, Twerenbold R, Mueller C, Biondi-Zoccai G, Waksman R . A systematic review and collaborative meta-analysis to determine the incremental value of copeptin for rapid rule-out of acute myocardial infarction. Am J Cardiol 2014; 113: 1581–1591.
Zhang X, Lu XM, Huang LF, Ye H . Copeptin is associated with one-year mortality and functional outcome in patients with acute spontaneous basal ganglia hemorrhage. Peptides 2012; 33: 336–341.
Ntaios G, Faouzi M, Ferrari J, Lang W, Vemmos K, Michel P . An integer-based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. Neurology 2012; 78: 1916–1922.
Strbian D, Seiffge DJ, Breuer L, Numminen H, Michel P, Meretoja A, Coote S, Bordet R, Obach V, Weder B . Validation of the DRAGON score in 12 stroke centers in anterior and posterior circulation. Stroke 2013; 44: 2718–2721.
Koton S, Eizenberg Y, Tanne D, Grossman E . Trends in admission blood pressure and stroke outcome in patients with acute stroke and transient ischemic attack in a National Acute Stroke registry. J Hypertens 2016; 34: 316–322.
Josephson SA, Hills NK, Johnston SC . NIH Stroke Scale reliability in ratings from a large sample of clinicians. Cerebrovasc Dis 2006; 22: 389–395.
Sato S, Toyoda K, Uehara T, Toratani N, Yokota C, Moriwaki H, Naritomi H, Minematsu K . Baseline NIH Stroke Scale Score predicting outcome in anterior and posterior circulation strokes. Neurology 2008; 70: 2371–2377.
Bustamante A, Garcia-Berrocoso T, Llombart V, Simats A, Giralt D, Montaner J . Neuroendocrine hormones as prognostic biomarkers in the setting of acute stroke: overcoming the major hurdles. Expert Rev Neurother 2014; 14: 1391–1403.
Greisenegger S, Segal HC, Burgess AI, Poole DL, Mehta Z, Rothwell PM . Copeptin and long-term risk of recurrent vascular events after transient ischemic attack and ischemic stroke: population-based study. Stroke 2015; 46: 3117–3123.
Acknowledgements
We are deeply appreciative of the participants in this study and thank all staff for their support and assistance. This study was supported by the National Natural Science Foundation of China (Grant No. 81102189) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, Q., Tian, Y., Peng, H. et al. Copeptin as a biomarker for prediction of prognosis of acute ischemic stroke and transient ischemic attack: a meta-analysis. Hypertens Res 40, 465–471 (2017). https://doi.org/10.1038/hr.2016.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.165
Keywords
This article is cited by
-
Copeptin: a potential blood biomarker for acute ischemic stroke
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2021)
-
Serum Copeptin levels in the emergency department predict major clinical outcomes in adult trauma patients
BMC Emergency Medicine (2020)
-
Copeptin as a marker of outcome after cardiac arrest: a sub-study of the TTM trial
Critical Care (2020)
-
Blood Biomarkers for Stroke Diagnosis and Management
NeuroMolecular Medicine (2019)
-
Novel Biomarkers to Detect Target Organ Damage in Acute Hypertension
Current Hypertension Reports (2018)