Abstract
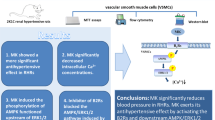
Angiotensin (Ang) II has an important role in the vascular smooth muscle cell (VSMC) proliferation and migration and subsequently in the development of vascular diseases, whereas dopamine has the opposite effect. Previous studies have shown an interaction between dopamine and AT1 receptors in the kidney. The dopamine D4 receptor is expressed in arteries and has an inhibitory effect on VSMC proliferation. We hypothesized that the D4 receptor, through its interaction with the AT1a receptor, may have an inhibitory effect on Ang II-mediated VSMC proliferation and migration, which could have a pivotal role in hypertension-induced vascular remodeling. In the current study, we found that Ang II markedly induced the proliferation and migration of A10 cells, which was inhibited by the D4 receptor agonist PD168077. The activation of the D4 receptor by PD168077 inhibited AT1a receptor expression in a concentration- and time-dependent manner. These effects were attenuated by silencing the D4 receptor with a D4 receptor-targeting small interfering RNA. The D4 receptor-mediated inhibition of AT1 receptor function involved protein kinase A (PKA). The activation of the D4 receptor by PD168077 increased PKA activity in A10 cells, and the presence of a PKA inhibitor (PKA inhibitor 14–22, 10−7 mol l−1 per 24 h) blocked the inhibitory effect of the D4 receptor on AT1 receptor expression and function. The inhibitory effect of the D4 receptor on AT1 receptor expression and function was preserved in VSMCs (primary culture) from spontaneously hypertensive rats relative to VSMCs from Wistar-Kyoto rats. In conclusion, our data provide insight into the regulatory role of the D4 receptor on AT1a receptor expression and function in VSMCs and suggest that targeting the action of the D4 receptor may represent an effective therapeutic approach for the treatment of cardiovascular diseases.
Similar content being viewed by others
Introduction
Vascular smooth muscle cells (VSMCs) are critically involved in vascular diseases. The abnormal proliferation and migration of VSMCs participate in smooth muscle hypertrophy, which is often associated with many vascular diseases, including hypertension, atherosclerosis and restenosis after balloon angioplasty.1, 2 Angiotensin II (Ang II), as the major effector of the renin–angiotensin system, has an important role in regulating blood pressure and body fluid volume. Moreover, Ang II, via the AT1a receptor, is a critical regulator of rat VSMC proliferation and migration, which are essential in the vascular remodeling associated with hypertension.3, 4, 5, 6
Dopamine receptors exert their beneficial effects on blood pressure homeostasis through the regulation of epithelial sodium transport and vascular smooth muscle tone. Dopamine receptors are classified into the D1-like and D2-like subtypes based on their structure and pharmacology. D1-like receptors are composed of D1 and D5 receptors, whereas D2-like receptors are composed of D2, D3 and D4 receptors.7, 8, 9, 10, 11 As a major component of the D2-like receptor subfamily, the D4 receptor has been shown to have an important role in the pathogenesis of hypertension. D4 receptor-deficient mice have higher blood pressure than wild-type mice.9, 12 There is increasing evidence of an interaction between dopamine receptors and the AT1 receptor.12, 13, 14, 15 Our previous study showed a negative interaction between D3 and AT1 receptors whereby the activation of the D3 receptor inhibits AT1 receptor expression and function in renal proximal tubule cells.15 The disruption of the D4 dopamine receptor gene in mice produces hypertension that is associated with increased renal AT1 receptor expression. The hypotensive effect of a bolus intravenous injection of the AT1 receptor antagonist losartan lasted longer in D4 receptor-deficient mice than their wild-type littermates.12 The D4 receptor is expressed in VSMCs and activation of the D4 receptor inhibits insulin-mediated VSMC proliferation and migration.16 We hypothesized that the D4 receptor, by interacting with the AT1a receptor, may have an inhibitory effect on Ang II-mediated VSMC proliferation and migration and could therefore play a pivotal role in hypertension-induced vascular remodeling. To test this hypothesis, we used immortalized rat aortic smooth muscle cells (A10) and primary cultures of aortic VSMCs from Wistar-Kyoto (WKY) and spontaneously hypertensive rats (SHRs) to determine whether the D4 receptor affects Ang II-induced proliferation and migration of VSMCs and investigated possible signaling pathways of such an interaction.
Methods
Materials
PD168077, L745870 (a dopamine D4 receptor antagonist) and Ang II were purchased from Sigma (Sigma, St Louis, MO, USA). Rabbit polyclonal AT1 receptor antibody and monoclonal α-actin antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). PKA inhibitor 14–22 was purchased from Calbiochem (Darmstadt, Germany). SDS-polyacrylamide gels were from Pierce (Rockford, IL, USA). Polyvinylidene fluoride (PVDF) and protein gel apparatus were purchased from Bio-Rad (Hercules, CA, USA). Minimal essential medium (MEM), Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco/Invitrogen (Carlsbad, CA, USA); fibroblast growth factor (FGF), epidermal growth factor (EGF), phosphate-buffered saline (PBS), penicillin/streptomycin and non-essential amino acids were purchased from Sigma.
Cell culture
A10 cells,16, 17 a smooth muscle cell line from rat thoracic aorta, were purchased from ATCC (A10; ATCC, Hercules, CA, USA). Primary VSMCs were isolated from the aortae of 8-week-old male (200–220 g) SHRs and age-matched WKY rats (Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences, Beijing, China) using a digestion method.18, 19 VSMCs were grown in 10% FBS-DMEM containing 1% antibiotics and incubated in a CO2 incubator (95% CO2, 37 °C). After reaching 70% confluence, the VSMCs were incubated in serum-free DMEM for 16–24 h prior to use.
VSMC proliferation assay
VSMC proliferation was quantified by measuring the uptake of tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT) and using cell counting assays. The cells were seeded into 96-well (100 μl of medium per well) culture plates at a density of 1 × 104 cells per well, made quiescent for 24 h and then pretreated with PD168077 with or without L745870 for 30 min, followed by stimulated with various concentrations of Ang II for an additional 24 h. Subsequently, 20 μl of MTT (5 mg ml−1) were added to each well, and the incubation was continued for an additional 4 h at 37 °C. Next, dimethyl sulfoxide (DMSO, 150 μl) was added to each well, and absorbance was read at 490 nm on a microplate reader (model 680, Bio-Rad). The growth of the VSMCs was examined by cell counting. The cells were made quiescent by serum starvation in serum-free medium, then pretreated with PD168077 with or without L745870 for 30 min and stimulated with Ang II for an additional 24 h. After incubation, the cells were counted in a hemocytometer (trypan blue uptake, which indicates cell death, was observed in <10% of the cells). Each count is an average of three repeat samplings, and each data point is the average of three experiments.16, 17
VSMC migration
Cell migration was examined using transwell and scratch-wound migration assays.16, 20 The transwell migration assay was performed using 24-well tissue culture plates (BD Bioscience, Becton, NJ, USA) with an 8-μm-pore polycarbonate membrane. The number of migratory cells was counted in 10 randomly chosen fields of duplicate chambers at a magnification of × 200 for each sample. For the scratch-wound migration assay, VSMCs were seeded in a six-well plate at a density of 1 × 105 cells per well, grown to confluence, starved for 24 h and then pretreated with PD168077 with or without L745870 for 30 min and stimulated with Ang II for an additional 24 h. The cell monolayer was scratched with a small tip along the ruler and allowed to recover for 24 h in fresh starvation medium (serum-free DMEM) for 48 h. The cells were visualized using an Olympus IX-70 inverted microscope (Olympus, Tokyo, Japan). The migration area (%) was analyzed in 10 randomly chosen fields under an inverted microscope by using NIH Image J software, area at 0 h pre area at 48 h × 100% was calculated.
Immunoblotting
A10 cells were treated with vehicle (dH2O), PD168077 or L745870 at the indicated concentrations and times. After treatment, the A10 cells were washed once in PBS and lysed in a lysis buffer. Extraction of proteins, electrophoresis, transfer, immunodetection and densitometric evaluation were performed as previously described.15, 16, 17, 21 The amount of protein transferred onto the membranes was normalized by immunoblotting with α-actin (1:400).
Transfection with D4 receptor-targeting siRNA
Knockdown of the D4 receptor with siRNA was accomplished in A10 cells by transfection with 2.5 -μl D4 receptor-targeting siRNA or scrambled siRNA (scRNA) for 48 h, using Lipofectamine 2000 reagent (Invitrogen).22, 23 After transfection, quiescent VSMCs were pretreated with PD168077 for 30 min and then stimulated with Ang II for an additional 24 h prior to immunoblotting, proliferation and migration assays.16, 17, 20
RT-PCR and real-time quantitative PCR
Total RNA from A10 cells was isolated using a Trizol procedure (Invitrogen). Total RNA (2 μg) was used to synthesize the cDNA, which served as a template for the amplification of AT1a receptor and β-actin (housekeeping gene). Primer sequences for AT1a receptor were 5′-AAAGGGCAAGGAACCTTTGT-3′ (forward) and 5′-CAGATGCGAAATAACGCAGA-3′ (reverse). Primer sequences for β-actin were 5′-GTGGGTATGGGTCAGAAGGA-3′ (forward) and 5′-AGCGCGTAACCCT CATAGAT-3′ (reverse). The amplification was performed under the following conditions: 94 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 45 s. This was followed by a final extension at 72 °C for 10 min. The PCR products were electrophoresed in 2% agarose gels.16, 17 AT1a mRNA was quantified by real-time quantitative (q) RT-PCR using SYBR green PCR technology (Applied Biosystems, Foster City, CA, USA) with the following conditions: 10 s at 95 °C; 45 s at 62 °C; 60 s at 72 °C, repeated for 40 cycles.23, 24 Each qRT-PCR sample was normalized by the expression of ribosomal protein S16 and is expressed as fold change. Primer sequences of AT1a receptor and 16S used for qRT-PCR are follows: AT1a receptor forward 5′-GCGGTCTCCTTTTGATTTCC-3′, reverse 5′-CAAAGGGCTCCTGAAACTTG-3′ (reverse). 16S forward 5′-ATCTCAAAGGCCCTGGTAGC-3′, reverse 5′-ACAAAGGTAAACCCCGATCC-3′.
Measurement of PKA activity
PKA activity was measured using SignaTECT cAMP-dependent PKA assay (Promega, Southampton, UK), which utilizes biotinylated kemptide (LRRASLG), a peptide substrate derived from the in vivo substrate pyruvate kinase. A10 cells (5 × 106 cells) were pre-incubated with control buffer or with the D4 receptor agonist, PD168077 (10−7 mol l−1), for 30 min at 37 °C; the cells were washed with ice-cold phosphate-buffered saline once, followed by the complete removal of the buffer. PKA activity was measured by scintillation counting.17, 25, 26
Statistical analysis
The data are expressed as the mean±s.e.m.. Comparison within groups was based on one-way repeated measures ANOVA (or paired t-test when only 2 groups were compared), and comparison among groups (or t-test when only two groups were compared) was based on one-way factorial ANOVA with Holm–Sidak test. A value of P<0.05 was considered significant.
Results
Stimulation of the D4 receptor inhibits Ang II-mediated VSMC proliferation and migration
VSMC proliferation and migration have an important role in hypertension and atherosclerosis. To observe the effect of the D4 receptor on AT1 receptor function in arteries, we first examined the effects of PD168077, a D4 receptor agonist, on Ang II-induced proliferation of A10 cells, as determined by MTT uptake and cell counting. Ang II stimulated VSMC proliferation of the A10 cells in a concentration-dependent manner (10−8–10−6 mol l−1) (Figure 1a). Although PD168077 (10−10–10−6 mol l−1), by itself, had no effect on cell proliferation (Figure 1b), it reduced the Ang II (10−7 mol l−1 per 24 h)-mediated VSMC proliferation in a concentration-dependent manner (10−10–10−6 mol l−1) (Figures 1c and d). The inhibitory effect of the D4 receptor was specific because in the presence of D4 receptor antagonist L745870 (10−7 mol l−1 per 24 h), the effect of PD168077 was blocked (Figures 1e and f).
Effect of the D4 receptor on Ang II-induced proliferation in A10 cells. (a) Effect of Ang II on proliferation in A10 cells. Vascular smooth muscle cell proliferation was determined by the uptake of 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide after incubation with varying concentrations (10−10 –10−6 mol l−1) of Ang II (n=6, *P<0.05 vs control). (b) Effect of a D4 receptor agonist, PD168077, on proliferation in A10 cells. A10 cell proliferation was determined by the uptake of 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide after incubation with varying concentrations of PD168077 (10−10–10−6 mol l−1) (n=10). (c and d) Effect of a D4 receptor agonist, PD168077, on Ang II-mediated proliferation in A10 cells. A10 cell proliferation was determined by the uptake of 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (c, n=8, *P<0.05 vs Ang II alone) or cell number (d, n=9, *P<0.05 vs Ang II alone) after incubation with the indicated concentrations of Ang II (10−7 mol l−1) with or without the presence of PD168077 (PD, 10−10–10−6 mol l−1). (e and f). D4 receptor specificity on the proliferation in A10 cells. A10 cells were incubated with the indicated reagents (Ang II, 10−7 mol l−1; PD, PD168077, 10−7 mol l−1; L, L745870, 10−7 mol l−1) for 24 h. A10 cell proliferation was determined by the uptake of 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (e, n=8, *P<0.05 vs control; #P<0.05 vs Ang II alone) or cell number (f, n=8, *P<0.05 vs control; #P<0.05 vs Ang II alone).
We also studied the effects of Ang II and PD168077 on VSMC migration by transwell and scratch-wound migration test. The results showed that Ang II significantly increased VSMC migration. PD168077 alone had no effect, but inhibited the Ang II-mediated VSMC migration (Figures 2a and b). Consistent with the proliferation study, the inhibitory effect was specific because in the presence of the D4 receptor antagonist L745870 (10−7 mol l−1 per 24 h), the effect of PD168077 was blocked (Figures 2a and b).
Effect of D4 receptor on Ang II-mediated vascular smooth muscle cell migration. A10 cells were pretreated with a D4 receptor agonist, (PD, PD168077, 10−7 mol l−1) with or without a D4 receptor antagonist (L, L745870, 10−7 mol l−1) for 30 min and then incubated with vehicle (control) or Ang II (10−7 mol l−1) for an additional 24 h. A10 cell migration was determined by transwell (a) and scratch-wound migration assays (b). The results are expressed as migratory cell number (n=9, *P<0.05 vs control; #P<0.05 vs Ang II alone) or migration distance (n=9, *P<0.05 vs control; #P<0.05 vs Ang II alone). A full color version of this figure is available at Hypertension Research online.
Knockdown of the D4 receptor by a D4 receptor-targeting siRNA inhibits Ang II-mediated VSMC proliferation and migration
To specifically determine the involvement of the D4 receptor, excluding any D2 or D3 receptor involvement, in the Ang II-mediated VSMC proliferation and migration, we studied the effect of Ang II and PD168077 on VSMC proliferation and migration after knockdown of the D4 receptor by a D4 receptor-targeting siRNA. As shown in Figures 3a and b, in A10 cells transfected with a D4 receptor siRNA, the effect of Ang II was enhanced and minimized the PD168077-mediated inhibition of Ang II-induced proliferation and migration, confirming the inhibitory effect of D4 receptors on Ang II-induced proliferation and migration. The inhibitory effect of D4 receptor-targeting siRNA on D4 receptor expression is shown in Supplementary Figure S1.
Inhibition of the D4 receptor on Ang II-mediated vascular smooth muscle cell proliferation and migration after knockdown of D4 receptors by a D4 receptor-targeting siRNA. Vascular smooth muscle cells were transfected with a D4 receptor-targeting siRNA (50 nmol l−1, 48 h) or scrambled siRNA (50 nmol l−1, 48 h) and then treated with vehicle (control), Ang II (10−7 mol l−1) alone or in the presence of a D4 receptor agonist (PD, PD168077, 10−7 mol l−1) (Ang II +PD). A10 cell proliferation was determined by the uptake of 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (a, n=9, *P<0.05 vs control; #P<0.05 vs Ang II alone). A10 cell migration was determined by transwell assay (b, n=6, *P<0.05 vs control; #P<0.05 vs Ang II alone).
Activation of the D4 receptor decreases AT1a receptor expression in A10 cells
Ang II increases rat VSMC proliferation and migration primarily through the occupation of the AT1a receptors subtype. To determine the mechanism underlying the negative regulation of Ang II function by the D4 receptor, we examined the expression of the AT1 receptor after treatment with a D4 receptor agonist. The results showed that PD168077-mediated activation of the D4 receptor decreased AT1 receptor expression in a concentration (10−10–10−6 mol l−1)- and time (4–30 h)-dependent manner (Figures 4a and b ). The effect of the D4 receptor on the AT1 receptor expression was exclusively because of the D4 receptor itself because pretreating the A10 cells with the D4 receptor antagonist L745870 (10−7 mol l−1 per 24 h) or with a D4 receptor-targeting siRNA blocked the effects of PD168077 on AT1 receptor expression (Figures 4c and d). To determine whether the D4 receptor-induced AT1 receptor downregulation involves regulation of protein stability, A10 cells were pretreated with 100 μM of cycloheximide, stimulated with PD168077 (10−7 mol l−1) and harvested at different time points (see Supplementary Material). Then, AT1 receptor protein was assayed by immunoblotting. The data indicated that AT1 protein stability was not affected by treatment with PD168077 in A10 cells (Supplementary Figure S2).
Effect of D4 receptor on AT1 receptor expression in A10 cells. (a) Concentration-dependent effect of D4 receptor on AT1 receptor protein expression in A10 cells. A10 cells were incubated with varying concentrations of the D4 receptor agonist PD168077 (PD, 10−10–10−6 mol l−1) for 24 h. AT1 receptor expression was quantified by immunoblotting. Distilled water, instead of PD168077, was used as control. The results are expressed as the ratio of AT1 receptor to α-actin densities (n=5, *P<0.05 vs control). (b) Time-course of the effect of D4 receptor on AT1 receptor protein expression in A10 cells. The cells were incubated at the indicated times with PD168077 (PD, 10−7 mol l−1). Distilled water, instead of PD168077, was used as control. The results are expressed as the ratio of AT1 receptor to α-actin densities (n=5, *P<0.05 vs control). (c and d) Specificity of D4 receptor effect on AT1 receptor expression in A10 cells. The AT1 receptor protein expressions were quantified by immunoblotting. A10 cells were incubated with the indicated reagents: (dH2O [Control], PD, PD168077, 10−7 mol l−1, L, L745870, 10−7 mol l−1 or PD+L) for 24 h (c). A10 cells were transfected with a D4 receptor-targeting siRNA (50 nmol l−1, 48 h) or scrambled siRNA (50 nmol l−1, 48 h) and then treated with vehicle (control) or D4 receptor agonist (PD, PD168077, 10−7 mol l−1) for 24 h. (d) (n=5, *P<0.05 vs scrambled siRNA control, #P<0.05 vs scrambled siRNA+PD). (e and f) The AT1a receptor mRNA expression was determined by RT-PCR (e) or real-time quantitative RT-PCR (f). The results are expressed as the ratio of AT1a receptor to β-actin densities for RT-PCR (n=7, *P<0.05 vs others) or S16 for real-time quantitative RT-PCR individually (n=6, *P<0.05 vs others).
The relative abundance of AT1a receptor mRNA was detected by RT-PCR (Figure 4e) and real-time qRT-PCR (Figure 4f). A10 cells were incubated with PD168077(10−7 mol l−1 per 24 h) with or without L745770 (10−7 mol l−1 per 24 h). The results showed that the activation of the D4 receptor, by PD168077 (10−7 mol l−1 per 24 h), decreased AT1a receptor mRNA expression, which was blocked in the presence of L745870, suggesting that the regulation of D4 receptor on AT1a receptor occurs at the transcriptional, post-transcriptional and translational levels.
Determination of the signaling pathways involved in the negative D4 receptor effect on AT1 receptor expression and function
To determine the underlying mechanism of the negative regulation of AT1 receptor expression by the D4 receptor, we determined whether there is direct interaction between these two receptors. Although we found co-localization between D4 and AT1 receptors (Supplementary Figure S3), there was no co-immunoprecipitation (data not shown).
We have previously shown that PKA is involved in the negative regulation of insulin receptor expression and function by the D3 receptor.17 The negative regulation of AT1 receptor function by the D4 receptor, being one of the subtypes of the D2-like receptor subfamily, may also involve PKA. We initially determined the activity of PKA in VSMCs after treatment with PD168077. We found that PD168077 increased PKA activity in A10 cells (Figure 5a). Activation of the D4 receptor (PD168077, 10−7 mol l−1) inhibited AT1 receptor expression. However, in the presence of the PKA inhibitor (PKA inhibitor 14–22, 10−7 mol l−1 per 24 h), the inhibitory effect of the D4 receptor on AT1 receptor expression was blocked (Figure 5b). To determine the role of PKA in the inhibitory effect of the D4 receptor on AT1 receptor function, we assessed the proliferation and migration of A10 cells using the MTT and transwell assays. Consistent with previous studies, stimulation with Ang II increased the proliferation and migration of A10 cells. Ang II-induced proliferation and migration of VSMCs were significantly suppressed by the D4 receptor agonist, PD168077. Pre-treatment with the PKA inhibitor (PKAI, 10−7 mol l−1) or D4 receptor antagonist, L745870 (10−7 mol l−1), blocked the inhibitory effects of PD168077 on Ang II-mediated VSMC proliferation and migration (Figures 5c and d). These results suggested that the PKA signaling pathway could be responsible for the D4 receptor-mediated negative regulation of AT1 receptor expression and function in VSMCs.
Role of protein kinase A in the inhibitory effect of PD168077 on AT1 receptor expression and function in A10 cells. A10 cells were treated with a D4 receptor agonist (PD168077, 10−7 mol l−1), a D4 receptor antagonist (L, L745870, 10−7 mol l−1) or both D4 receptor agonist (PD) and D4 receptor antagonist (L, L745870, 10−7 mol l−1) for 24 h. The activity of protein kinase A was determined by cAMP-dependent protein kinase A assay (a, n=6, *P<0.05 vs others). The inhibitory effect of D4 receptor agonist, PD168077 (PD, 10−7 mol l−1) on AT1 receptor expression was blocked in the presence of protein kinase A inhibitor 14–22 (PKAI, 10−7 mol l−1). The immunoblotting density was normalized by α-actin density (b, n=6, *P<0.05 vs others). The inhibitory effect of a D4 receptor agonist, PD168077 (PD, 10−7 mol l−1), on Ang II (10−7 mol l−1)-mediated vascular smooth muscle cell proliferation and migration was abolished in the presence of PKA inhibitor 14–22 (PKAI, 10−7 mol l−1). Vascular smooth muscle cell proliferation was determined by the uptake of 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (c, n=5, *P<0.05 vs control; #P<0.05 vs Ang II alone). Vascular smooth muscle cell migration was determined by transwell assay (d, n=5, *P<0.05 vs control; #P<0.05 vs Ang II alone).
Effect of the D4 receptor on AT1 receptor-mediated proliferation and migration in aortic VSMCs from WKY and SHRs
To determine whether the inhibition of the D4 receptor on AT1 receptor-mediated proliferation and migration of VSMCs had any physiological significance in hypertension, we compared the proliferative and migratory capacities of An II in primary cultures of aortic VSMCs from SHRs and WKY rats. The basal levels of proliferation were higher in VSMCs from SHRs than those from WKY rats, although Ang II significantly induced greater MTT uptake and a higher rate of migration in VSMCs from both SHRs and WKY rats (Figures 6a and b). PD168077 significantly attenuated the stimulatory effect of Ang II on VSMC proliferation (WKY:32.1±4.3% vs SHR:29.1±3.6%) and migration (WKY:46.0±6.4% vs SHR:39.4±6.9%) in both SHRs and WKY rats. The inhibitory effect of PD168077 did not show any significant difference between the strains. Additionally, we evaluated the expression of the AT1 receptor in VSMCs from SHR and WKY rats after treatment with PD168077 (10−7 mol l−1 for 24 h). As shown in Figure 6c, the basal level of AT1 receptor expression was significantly higher in VSMCs from SHRs than those from WKY rats. However, the D4 receptor significantly inhibited AT1 receptor expression in VSMCs from both SHRs and WKY rats. No difference was observed in the degree of inhibition of AT1 receptor expression by the D4 receptor between SHR and WKY cells (SHR:46.8±1.96% vs WKY:44.9±1.2%). These results indicated that the inhibitory effect of the D4 receptor in arteries is preserved in hypertension; therefore, the D4 receptor could be a possible target for reduction of VSMC proliferation and migration in hypertension and atherosclerosis.
Effect of the D4 receptor on AT1 receptor expression and function in vascular smooth muscle cells from Wistar-Kyoto rats and spontaneously hypertensive rats: the effect of PD168077 on the proliferation and migration of vascular smooth muscle cells from Wistar-Kyoto rats and spontaneously hypertensive rats in response to Ang II. The primary cultured vascular smooth muscle cells from Wistar-Kyoto rats and spontaneously hypertensive rats were stimulated with vehicle (control), Ang II (10−7 mol l−1), D4 receptor agonist (PD168077, 10−7 mol/l), or Ang II (10−7 mol l−1) for 24 h. The proliferation of vascular smooth muscle cells was measured by the uptake of 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (a, n=6, *P<0.05 vs control; #P<0.05 vs Ang II alone). Vascular smooth muscle cells migration was measured by transwell assay (b, n=6, *P<0.05 vs control; #P<0.05 vs Ang II alone). The effect of PD168077 on AT1 expression in vascular smooth muscle cells from Wistar-Kyoto rats and spontaneously hypertensive rats was quantified by immunoblotting (c, n=6, *P<0.05 vs control; #P<0.05 vs Wistar-Kyoto control).
Discussion
Ang II contributes to vascular lesions by promoting VSMC proliferation and migration. However, the mechanism remains to be defined. Multiple pieces of evidence strongly implicate the action of Ang II through the AT1a receptor, as the mediator of VSMC proliferation and migration that are important in the development of atherosclerosis and hypertension. Thus, the inhibition of VSMC proliferation or migration may potentially be an important therapeutic strategy for the treatment of hypertension.6, 24, 27, 28, 29
Several studies have shown that the dopamine and AT1 receptors interact to regulate sodium and water balance in the kidney.13, 14, 15, 30 AT1 receptors stimulate renal proximal tubular ion-transporting proteins that are inhibited by D1-like receptors and therefore, the natriuretic effect of D1-like receptors is enhanced when AT1 receptors are blocked.13 The D1-like receptor agonist fenoldopam decreases AT1 receptor expression in renal proximal tubule cells from WKY rats and SHRs.13 Increases in [Na+]i led to a higher number of D1 receptors in the plasma membrane that is paralleled by a reduced abundance of AT1 receptors in the opossum kidney (OK) epithelial cell line.30 Exposure of rat renal proximal tubule cells to a D1 receptor agonist resulted in a rapid partial internalization of the AT1 receptor and complete inhibition of AT1 receptor signaling.13 D3−/− mice have renin-dependent hypertension and renal AT1 receptor function is greater in D3−/−mice than their wild-type (D3+/+) littermates.31 Activation of the D3 receptor decreases AT1 receptor expression in renal proximal tubule cells from normotensive rats.15 The protein expression of the AT1 receptor is also increased in homogenates of the kidney of D4−/− mice relative to their D4+/+ littermates.12 However, whether there is an interaction between D4 and AT1 receptors in resistance arteries has not been reported. We now report an interaction between D4 and AT1a receptors in A10 cells. The stimulation of D4 receptors decreased AT1a receptor mRNA and protein expression but did alter AT1 receptor protein stability. Therefore, the D4 receptor-mediated negative regulation of AT1a receptor expression may be exerted at the transcriptional, post-transcriptional and translational levels. However, the potential mechanisms by which the D4 receptor downregulates AT1a receptor gene transcription or AT1a receptor mRNA stability remain to be investigated.
Previous studies have shown that stimulation of the D4 or D3 receptor inhibits insulin-mediated VSMC proliferation.16, 17 The D4 receptor decreases the expression of AT1 receptors in VSMCs. However, whether the D4 receptor has an inhibitory effect on Ang II-mediated VSMC proliferation and migration is not known. In this report, we showed that activation of the D4 receptor inhibited the proliferation and migration of VSMCs that were induced by Ang II, although stimulation of the D4 receptor by itself had no effect. Transfection of VSMCs with D4 receptor-targeting siRNA almost completely prevented the inhibitory effect of D4 receptor activation by PD168077 on Ang II-induced proliferation and migration. This result, together with the ability of a D4 receptor antagonist to minimize also the effect of a D4 receptor agonist, confirmed that the D4 receptor can antagonize the ability of Ang II to induce VSMC proliferation and migration.
Our previous study showed that PKA is involved in the negative regulation of VSMC insulin receptor expression by the D3 receptor,17 another subtype in the D2-like receptor subfamily. Therefore, we sought to determine whether PKA is similarly involved in the negative regulation of the AT1 receptor by the D4 receptor. In the current study, we found that PKA activity in VSMCs was increased after treatment with the D4 receptor agonist PD168077. A PKA inhibitor prevented the inhibitory effect of the D4 receptor on AT1 receptor protein expression as well as the VSMC proliferation and migration mediated by Ang II, which suggested that the PKA signaling pathway is involved in the regulation of D4 receptor on AT1 receptor expression and function. Although, D2-like receptors are generally associated with inhibition of the PKA pathway, under certain circumstances, in some cells, D2-like receptors stimulate adenylyl cyclase activity.32, 33, 34
Dopamine receptor function, especially that of the D1 and D3 receptors, is impaired in the kidneys from hypertensive patients and SHRs,31, 35, 36 whereas their effects are preserved in arteries. Our previous study showed that activation of the D1 or D3 receptor inhibits alpha1-adrenergic receptor-mediated proliferation in VSMCs.37 The current study also found that the inhibitory effects of the D4 receptor on AT1 receptor expression and function are preserved in primary culture of VSMCs from WKY and SHRs.
In conclusion, our data provide insight into the negative regulation of AT1a receptor expression and function by the D4 receptor in VSMCs and suggest that targeting the action of the D4 receptor may represent an effective therapeutic approach in the treatment of cardiovascular diseases.
References
Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB . The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 2012; 95: 194–204.
Natarajan R, Rosdahl J, Gonzales N, Bai W . Regulation of 12-lipoxygenase by cytokines in vascular smooth muscle cells. Hypertension 1997; 30: 873–879.
Ozasa Y, Akazawa H, Qin Y, Tateno K, Ito K, Kudo-Sakamoto Y, Yano M, Yabumoto C, Naito AT, Oka T, Lee JK, Minamino T, Nagai T, Kobayashi Y, Komuro I . Notch activation mediates angiotensin II-induced vascular remodeling by promoting the proliferation and migration of vascular smooth muscle cells. Hypertens Res 2013; 6: 859–865.
Sanada F, Taniyama Y, Iekushi K, Azuma J, Okayama K, Kusunoki H, Koibuchi N, Doi T, Aizawa Y, Morishita R . Negative action of hepatocyte growth factor/c-Met system on angiotensin II signaling via ligand-dependent epithelial growth factor receptor degradation mechanism in vascular smooth muscle cells. Circ Res 2009; 105: 667–675.
Takeuchi K, Yamamoto K, Ohishi M, Takeshita H, Hongyo K, Kawai T, Takeda M, Kamide K, Kurtz TW, Rakugi H . Telmisartan modulates mitochondrial function in vascular smooth muscle cells. Hypertens Res 2013; 36: 433–439.
Ikeda Y, Takeuchi K, Kato T, Taniyama Y, Sato K, Takahashi N, Sugawara A, Ito S . Transcriptional suppression of rat angiotensin AT1a receptor gene expression by interferon-gamma in vascular smooth muscle cells. Biochem Biophys Res Commun 1999; 262: 494–498.
Harris RC, Zhang MZ . Dopamine, the kidney, and hypertension. Curr Hypertens Rep 2012; 14: 138–143.
Horita S, Seki G, Yamada H, Suzuki M, Koike K, Fujita T . Roles of renal proximal tubule transport in the pathogenesis of hypertension. Curr Hypertens Rev 2013; 9: 148–155.
Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA . Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol 2008; 294: H551–H569.
Banday AA, Lokhandwala MF . Dopamine receptors and hypertension. Curr Hypertens Rep 2008; 10: 268–275.
Lu Q, Yang Y, Villar VA, Asico L, Jones JE, Yu P, Li H, Weinman EJ, Eisner GM, Jose PA . D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hypertens Res 2013; 36: 684–690.
Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P . Jose PA.Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension 2006; 47: 288–295.
Zeng C, Luo Y, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA . Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension 2003; 42: 787–792.
Khan F, Spicarová Z, Zelenin S, Holtbäck U, Scott L, Aperia A . Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol 2008; 295: F1110–F1116.
Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA . Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res 2006; 99: 494–500.
Yu C, Wang Z, Han Y, Liu Y, Wang WE, Chen C, Wang H, Jose PA, Zeng C . Dopamine D4 receptors inhibit proliferation and migration of vascular smooth muscle cells induced by insulin via down-regulation of insulin receptor expression. Cardiovasc Diabetol 2014; 13: 97.
Huang H, Han Y, Wang X, Chen C, Yu C, He D, Wang H, Zhou L, Asico LD, Jose PA, Zeng C . Inhibitory effect of the D3 dopamine receptor on insulin receptor expression and function in vascular smooth muscle cells. Am J Hypertens 2011; 24: 654–660.
Kim HY, Jeong DW, Park HS, Lee TY, Kim HS . Comparison of 12-lipoxygenase expression in vascular smooth muscle cells from old normotensive Wistar-Kyoto rats with spontaneously hypertensive rats. Hypertens Res 2013; 36: 65–73.
Liu G, Hitomi H, Hosomi N, Lei B, Pelisch N, Nakano D, Kiyomoto H, Ma H, Nishiyama A . Mechanical stretch potentiates angiotensin II-induced proliferation in spontaneously hypertensive rat vascular smooth muscle cells. Hypertens Res 2010; 33: 1250–1257.
Garg N, Goyal N, Strawn TL, Wu J, Mann KM, Lawrence DA, Fay WP . Plasminogen activator inhibitor-1 and vitronectin expression level and stoichiometry regulate vascular smooth muscle cell migration through physiological collagen matrices. J Thromb Haemost 2010; 8: 1847–1854.
Chen K, Fu C, Chen C, Liu L, Ren H, Han Y, Yang J, He D, Zhou L, Yang Z, Zhang L, Jose PA, Zeng C . Role of GRK4 in the regulation of arterial AT1 receptor in hypertension. Hypertension 2014; 63: 289–296.
Yuen EY, Yan Z . Dopamine D4 receptors regulate AMPA receptor trafficking and glutamatergic transmission in GABAergic interneurons of prefrontal cortex. J Neurosci 2009; 29: 550–562.
Lemarié CA, Simeone SM, Nikonova A, Ebrahimian T, Deschênes ME, Coffman TM, Paradis P, Schiffrin EL . Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res 2009; 105: 852–859.
Xiao F, Puddefoot JR, Barker S, Vinson GP . Mechanism for aldosterone potentiation of angiotensin II-stimulated rat arterial smooth muscle cell proliferation. Hypertension 2004; 44: 340–345.
Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J, Rashid S, Mancino MG, LeSage G, Alpini G . Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol 2003; 284: G683–G694.
Fetalvero KM, Shyu M, Nomikos AP, Chiu YF, Wagner RJ, Powell RJ, Hwa J, Martin KA . The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol 2006; 290: H1337–H1346.
Gabor A, Leenen FH . Central mineralocorticoid receptors and the role of angiotensin II and glutamate in the paraventricular nucleus of rats with angiotensin II-induced hypertension. Hypertension 2013; 61: 1083–1090.
Shen YJ, Zhu XX, Yang X, Jin B, Lu JJ, Ding B, Ding ZS, Chen SH . Cardamonin inhibits angiotensin II-induced vascular smooth muscle cell proliferation and migration by downregulating p38 MAPK, Akt, and ERK phosphorylation. J Nat Med 2014; 68: 623–629.
Yamamoto K, Ohishi M, Ho C, Kurtz TW, Rakugi H . Telmisartan-induced inhibition of vascular cell proliferation beyond angiotensin receptor blockade and peroxisome proliferator-activated receptor-gamma activation. Hypertension 2009; 54: 1353–1359.
Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH . Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem 2003; 278: 28719–28726.
Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA . Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest 1998; 102: 493–498.
Obadiah J, Avidor-Reiss T, Fishburn CS, Carmon S, Bayewitch M, Vogel Z, Fuchs S, Levavi-Sivan B . Adenylyl cyclase interaction with the D2 dopamine receptor family; differential coupling to Gi, Gz, and Gs. Cell Mol Neurobiol 1999; 19: 653–664.
Watts VJ, Neve KA . Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Mol Pharmacol 1997; 52: 181–186.
Jackson CR, Chaurasia SS, Hwang CK, Iuvone PM . Dopamine D4 receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. Eur J Neurosci 2011; 34: 57–64.
Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA . Impaired renal D(1)-like and D(2)-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol 2001; 281: R1071–R1078.
Zeng C, Wang D, Asico LD, Welch WJ, Wilcox CS, Hopfer U, Eisner GM, Felder RA, Jose PA . Aberrant D1 and D3 dopamine receptor transregulation in hypertension. Hypertension 2004; 43: 654–660.
Li Z, Yu C, Han Y, Ren H, Shi W, Fu C, He D, Huang L, Yang C, Wang X, Zhou L, Asico LD, Zeng C, Jose PA . Inhibitory effect of D1-like and D3 dopamine receptors on norepinephrine-induced proliferation in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 2008; 294: H2761–H2768.
Acknowledgements
These studies were supported by grants from the National Science Foundation of China (81370354, 31130029), National Basic Research Program of China (2012CB517801) and National Institutes of Health (HL P01HL074940).
Author Contributions
CY and JC performed most of the experiments, analyzed the data and wrote the manuscript. WG and YH performed the cell counting experiments. WEW, XW and PAJ reviewed and edited the manuscript. HW and CZ designed the experiments and wrote and edited the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Rights and permissions
About this article
Cite this article
Yu, C., Chen, J., Guan, W. et al. Activation of the D4 dopamine receptor attenuates proliferation and migration of vascular smooth muscle cells through downregulation of AT1a receptor expression. Hypertens Res 38, 588–596 (2015). https://doi.org/10.1038/hr.2015.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.48
Keywords
This article is cited by
-
The dopamine receptor D4 regulates the proliferation of pulmonary arteries smooth muscle in broilers by downregulating AT1R
Animal Diseases (2021)
-
Vasoplegia after implantation of a continuous flow left ventricular assist device: incidence, outcomes and predictors
BMC Anesthesiology (2018)
-
Vascular structural and functional changes: their association with causality in hypertension: models, remodeling and relevance
Hypertension Research (2017)
-
Activation of angiotensin II type 1 receptors increases D4 dopamine receptor expression in rat renal proximal tubule cells
Hypertension Research (2017)
-
Histone deacetyltransferase inhibitors Trichostatin A and Mocetinostat differentially regulate MMP9, IL-18 and RECK expression, and attenuate Angiotensin II-induced cardiac fibroblast migration and proliferation
Hypertension Research (2016)