Abstract
In the normotensive rat atrium, adenosine-5′-triphosphate and uridine-5′-triphosphate exert a biphasic effect consisting of an initial negative inotropic effect (NIE) followed by a subsequent positive inotropic effect (PIE). We comparatively studied these responses in normotensive Wistar rats (NWRs) and spontaneously hypertensive rats (SHRs). Compared with NWRs, the NIE responses in the atria were lower and the PIE responses were higher in SHRs. The P1 purinoceptor antagonist, D 8-cyclopentyl-1,3-dipropylxanthine, partially blocked the NIE responses of both ATP and UTP and mildly enhanced the PIE responses in both NWRs and SHRs. Furthermore, the P2 purinoceptor blockers suramin and pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium salt induced a pronounced block of the PIE responses in both atria types. The PIE responses to ATP were inhibited more efficiently by nifedipine. These responses were depressed by ryanodine and, to a lesser extent, carbonyl cyanide 3-chlorophenylhydrazone in SHR atria compared with NWR atria. The higher responses in SHR rats suggest the existence of an augmented endoplasmic reticulum Ca2+ store and faster mitochondrial Ca2+ cycling in SHR atria compared with NWR atria. These data support the hypothesis that a dysfunction of purinergic neurotransmission and enhanced sympathetic activity are contributing factors in the pathogenesis of hypertension.
Similar content being viewed by others
Introduction
Genetic, environmental and other factors, such as diet, sedentary life and smoking, have been implicated in the pathogenesis of primary hypertension.1 In addition, alterations of sympathoadrenal axis activity and the rate of catecholamine release have been implicated in the pathogenesis of essential hypertension. Their involvement is supported by the enhanced sympathetic nerve activity demonstrated using microneurography,2 the elevated plasma levels of norepinephrine and epinephrine in hypertension3, 4 and in spontaneously hypertensive rats (SHRs)5, 6, 7 and the sympathoadrenal axis interference caused by the use of antihypertensive agents.8 Recent observations from our laboratories indicating that chromaffin cells stimulated by acetylcholine demonstrate more sustained quantal catecholamine release with faster vesicle fusion kinetics and augmented quantal size in SHRs compared with normotensive Wistar rats (NWRs) also support these associations.9, 10 In addition, we have demonstrated that SHR chromaffin cells have higher basal cytosolic ([Ca2+]c) and mitochondrial calcium concentrations ([Ca2+]m) and that acetylcholine induces greater transient elevations in the SHR chromaffin cell [Ca2+]c compared with NWR chromaffin cells.11
As SHRs exhibit enhanced sympathetic activity, the subsequent positive chronotropic and inotropic effects exerted through β1 adrenoceptors should also be enhanced. However, the sympathetic drive to the heart also has an additionally relevant component mediated by the co-release of adenosine-5′-triphosphate (ATP) and uridine-5′-triphosphate (UTP) with norepinephrine,12 which also have relevant effects on cardiac inotropism.13 In the rat atrium, for instance, ATP and UTP first exert a negative inotropic effect (NIE) mediated by P1 purinoceptors that is curiously followed by a positive inotropic effect (PIE) associated with P2 purinoceptors.14, 15, 16
P1 purinoceptors are classified as A1, A2A, A2B14, 17 and A3.15 P2 purinoceptors are classified as P2X and P2Y;18 the former are coupled to non-selective cation channels, and the latter are coupled to G-proteins, which increase inositol triphosphate and induce Ca2+ mobilization from the endoplasmic reticulum.19, 20 Interestingly, changes in Ca2+ homeostasis have been found in various tissues of SHRs including the heart,21, 22 mesenteric and skeletal arteries23 and chromaffin cells.11 Furthermore, we have also found a lower functional expression of L-type voltage-dependent Ca2+ channels (α1D, Cav 1.3) that is compensated by a higher expression of P/Q voltage-dependent Ca2+ channels in the chromaffin cells of SHRs compared with NWRs; concomitantly, the modulation of the Ca2+ current by ATP24 was more pronounced in SHRs, in agreement with an observed 10-fold higher expression of the mRNA levels of the P2Y2 subtype of purinoceptors.25
The investigation presented herein was undertaken to comparatively explore the dual effects of ATP and UTP on atrial contractions in NWRs and SHRs and the effects of nifedipine (to block Ca2+ entry through voltage-dependent Ca2+ channels), ryanodine (to deplete the endoplasmic reticulum Ca2+ store) and the protonophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (to prevent mitochondrial Ca2+ uptake) on the identified dual effects.
Methods
Animals
Male SHRs and Wistar rats (normotensive controls-NWRs) of the same age (4–6 months) weighing 350–550 g were used. SHRs become frankly hypertensive, reaching stable mean arterial pressure values at ∼180 mm Hg, at 3 months of age.26, 27 All experimental procedures were approved by the Ethics Committee for the Use of Laboratory Animals of the Federal University of São Paulo, UNIFESP (0778/11).
Tissue isolation
The rats were euthanized by guillotine, and the hearts were rapidly removed. The left atria were dissected and mounted between platinum electrodes in 10 ml organ baths containing Krebs–Henseleit solution heated to 36.5 °C. One end of each atrium was attached to an FT202 force transducer (CB Sciences, USA) via a cotton thread to record isometric contractions. The transducer was connected to an ETH 400 amplifier (CB Sciences), which was coupled to a PowerLab digital data acquisition system (AD Instruments, Sydney, Australia), allowing the force of contraction to be recorded and stored during the experiment. The software used to convert analog data to digital data was Chart for Windows (v. 4.1.2, AD Instruments), which has a PC interface (Pentium II 266 MMX, 64 MB RAM). The Krebs–Henseleit solution had the following composition (in mM): NaCl 122.3, KCl 4.6, KH2PO4 1.2, MgSO4.7H2O 1.2, NaHCO3 17.4; CaCl2.H2O 1.5 and D-glucose 11.1, with a pH of 7.4. The solution was maintained at 36.5 °C and saturated with 95% O2 and 5% CO2.
Electric field stimulation
The left atria were stimulated by square-wave electrical pulses (2 Hz, 5 ms), and the voltage level (range 10–16 V) for stimulation was 20% greater than the atrial contraction threshold.16 The electric field was generated using platinum electrodes, which were diametrically opposed in the chamber and connected to a voltage electrical stimulator (Grass S48—Stimulator, Grass Inst. Div, Astro-Med Inc., West Warwick, RI, USA). Atria were equilibrated for 40 min before the experiments were started.16
Experimental protocol
After the equilibration period, each atria received a single dose of each agonist.16, 20 Non-cumulative concentration-response curves for ATP and UTP (1 nM to 1 mM each) were calculated in NWR and SHR atria to determine the concentration that produced 80% of the maximum effect (EC80). The EC80s of ATP and UTP were used in the following experimental protocols.
The agonists were also tested after incubation with the antagonists (20 min). The curves, in the absence or presence of the antagonists, were obtained on the same preparation.
In addition, the biphasic effect produced by ATP and UTP on atrial inotropism was investigated to confirm the hypothesis of P1 and P2 purinoceptor participation. Therefore, D 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (1 μM), a selective antagonist of A1 receptors, or suramin (100 μM) and pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium salt (PPADS) (10 μM), selective antagonists of P2 receptors, were preincubated for 20 min, and the inotropic effects of ATP and UTP were analyzed, as described by Froldi et al.16 and Gergs et al.28
To confirm the role of calcium in cardiac contraction, the left atria from hypertensive and control rats were used to evaluate the PIE induced by ATP and UTP in the presence or absence of an L-type calcium channel blocker (nifedipine; 0.1 μM), depletion of the endoplasmic reticulum calcium store (ryanodine, 3.0 nM) and mitochondrial depolarization (CCCP; 0.1 μM) following a preincubation period of 20 min.
Analysis of results
Blood pressure, body weight, ventricular weights, ratio of ventricular weight to body weight and left atria weight were compared between SHRs and NWRs using an unpaired t-test. In addition, we analyzed contractile parameters of the left atria, such as resting contraction, peak contraction, time to peak contraction, time to relax, peak rate of tension increase (+dT/dt) and peak rate of tension decrease (−dT/dt), in both animal strains.
Inotropic effects of the nucleotides were expressed as the Δ% of the basal force of contraction. Because both negative and positive effects were present (Figure 1), the PIE was expressed as the difference between the maximum PIE and the minimum value of contractility during the NIE divided by the basal force of contraction (value before the NIE). Therefore, both negative and positive effects were expressed as percentages of the same control value, as described by Froldi et al.16 and Gergs et al.28 The percent inhibition of the response was also calculated to compare the effects on calcium handling in NWRs and SHRs.
(a) Shows the scheme of purinergic neurotransmission in rat atria. ATP and/or UTP are co-released with noradrenaline (NOR) or acetylcholine (ACh). At the neuroeffector junction, ATP is degraded by nucleotidases, until adenosine (ADO) interacts with P1 receptors that are followed by an inhibition of adenylyl cyclase (AC) and, causing a decrease in the cAMP levels, whereas PKA activity diminish Ca2+ concentration, promoting a NIE. Moreover, ATP or UTP may activate the P2X or P2Y receptors followed by an increase of Ca2+ influx or phospholipase C/inositol triphosphate (PLPC/IP3) pathway, respectively, leading to PIE. (b) Shows the time course effect of a single concentration of ATP or UTP in atria. The latter exposure produced a biphasic effect represented by an initial NIE followed by a PIE. Individual vertical lines show atrial contractions elicited by field stimulation with pulses of 5 ms at 2 Hz. The basal contraction is known as baseline contraction amplitude (BCA). (c) Shows that NIE and PIE were calculated according to the formulas 1 and 2, respectively. (Figure based on Gergs et al.28).
Statistical analysis
Individual data are expressed as the mean±standard error of the mean (s.e.m.), and at least six experiments (n) were performed in each case. Mean values were compared using the conventional analysis of variance for paired samples (Bonferroni post-test) or were subjected to Student’s t-test. Statistical analysis was performed using GraphPad Instat (version 5.01, GraphPad Software Inc, San Diego, CA, USA). Statistically significant differences were considered at P<0.05.
Drugs used
The disodium salts of ATP and UTP, PPADS, suramin, DPCPX, nifedipine, ryanodine and CCCP were purchased from Sigma (St Louis, MO, USA). DPCPX was dissolved in dimethyl sulfoxide. The total volume of dimethyl sulfoxide added never exceeded 0.2% v v−1 of the organ bath. All other drugs mentioned were dissolved in Milli-Q purified water.
Results
SHRs that were 16–24 weeks of age exhibited a significant increase in mean arterial pressure accompanied by an increase in the ratio of ventricle weight to body weight compared with NWRs (Table 1). There was no difference in the parameters of isometric contractile performance of the left atria of SHRs and NWRs (Table 2).
As described previously,16 ATP and UTP exert a dual effect on rat atria. This is reflected by an initial NIE followed by a PIE. In the continuous presence of the purinoceptor agonist, the NIE developed slowly but gradually and plateaued within ∼1 min; subsequently, the tissue started to gradually recover its basal level of contraction and continued this increase for a few minutes to reach a stable plateau above the baseline contraction (Figures 2a, c). For the sake of clarity in the data analysis, we have provided a schematic of the dual nucleotide effects, the NIE component and the PIE component in Figure 1. We also show the formula used to estimate the normalized NIE and PIE values as the % of the initial baseline contraction amplitude.28
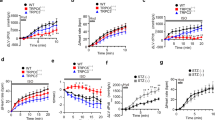
Effects of ATP at 300 μM (a) and UTP at 300 μM (c) on the basal contraction amplitude in electrically driven left atria of NWRs and SHRs. The ATP initially produced a NIE followed by PIE. (b, d) Concentration-response curves ATP and UTP (1 μM to 1 mM) in electrically driven left atria of SHR and NWR. The PIE responses to ATP were measured as indicated in Figure 1 and NIE response to ATP was calculated also as indicated in Figure 1 and expressed as a percentage of baseline amplitude of contractions. Each point represents the mean±s.e.m. from 7 to 20 experiments. (*P<0.05, difference between NWRs and SHRs, one-way analysis of variance (ANOVA), Bonferroni post-test).
Non-cumulative concentration-response curves for ATP and UTP in left atria isolated from NWRs and SHRs
As shown in Figures 2a, c, ATP and UTP exerted a biphasic effect on atrial inotropism. Both effects were tested at single concentrations in each preparation (1–1000 μM). For instance, at 300 μM, ATP and UTP initially produced a smaller NIE response in SHRs when compared with NWRs, as shown in the example record in Figure 2a. In addition, a greater PIE response followed the NIE in SHRs (Figure 2a).
The pooled results of the non-cumulative concentration-response curves for ATP and UTP showed that these nucleotides induced a diminished NIE response and an augmented PIE response in the left atria of SHRs compared with those of NWRs (Figures 2b, d). The maximum effects of both nucleotides were obtained at 1 mM.
The NIE response to 300 μM ATP was significantly decreased in SHRs (−46±2%) when compared with NWRs (−58±1%) (Figure 2b). In addition, the PIE response to ATP was increased in SHRs (46±2%) compared with NWRs (35±2%, P<0.05) (Figure 2b).
The NIE response to 300 μM UTP was decreased in SHRs (−19.7±0.9%) compared with NWRs (−27±2%, P<0.05). However, the PIE response to this nucleotide was increased in SHRs (33±1%) when compared with NWRs (40±3%, P<0.05).
To compare the effects of both nucleotides, a concentration of 300 μM was used for ATP and UTP, which corresponds to the EC80. Significant differences in the NIEs and PIEs were produced by ATP and UTP at 300 μM in the left atria of SHRs and NWRs (Figures 2b, d). The Emax values for the PIE response to both nucleotides are summarized in Table 3.
The corresponding pD2 value was significantly higher in ATP-stimulated SHR atria (4.60±0.07) when compared with NWR atria (4.30±0.08). The Emax was also significantly higher. Such alterations in pD2 were not observed in atria exposed to UTP, which induced alterations only in Emax.
Effects of P1 and P2 purinoceptor blockers on the atrial response of SHRs and NWRs to ATP and UTP
DPCPX (1 μM, preincubated for 20 min), a selective A1 purinoceptor antagonist,26, 27 did not by itself alter basal contraction during incubation for 20 min. The compound partially inhibited the NIE response to 300 μM ATP in SHR and NWR atria. Moreover, the PIE response to ATP was potentiated in the presence of DPCPX (Figure 3a). DPCPX also more effectively inhibited the NIE produced by ATP in the left atria of NWRs (49±2%) than in those of SHRs (35±2%, P<0.05) (Figure 3a).
Effects of A1 purinoceptor antagonist DPCPX on NIE and PIE response to 300 μM ATP and 300 μM UTP in electrically driven left atria of SHRs and NWRs. DPCPX 1 μM was incubated for 20 min before adding the nucleotides; the blocker did not alter baseline atrial contraction. Values are expressed as mean±s.e.m. of eight experiments, *P<0.05 difference between NWRs and SHRs, #P<0.05 difference between the inotropic response in presence and absence of antagonist (one-way ANOVA, Bonferroni post-test).
The presence of a biphasic effect of 300 μM UTP in the presence of DPCPX was also evaluated (Figure 3b). DPCPX blocked the NIE response by 41.0±0.8% in NWRs and 54±1% (P<0.05) in SHRs. However, DPCPX did not increase the PIE of UTP in NWRs or SHRs (Figure 3b).
To determine whether the PIE response was mediated by P2 purinoceptors, we tested the influence of 100 μM suramin29 on the nucleotide effects on atrial contractions. Suramin drastically antagonized the PIEs of ATP and UTP (Figures 4, 5). The PIE of ATP was inhibited by 55±1% in NWRs and 52.0±0.4% in SHRs when compared with the control response normalized to 100%. Furthermore, suramin potentiated the NIE response to ATP in SHRs by 46±4% (P<0.05) (Figure 4a) and more effectively inhibited the PIE response to UTP in SHRs compared with NWRs. This blockade amounted to 33±1% for NWRs and 41.0±0.9% SHRs (P<0.05). The NIE responses were unaltered by suramin in both SHRs and NWRs (Figure 4b).
Effects of the P2 purinoceptor antagonist suramin on the NIE and PIE response to 300 μM ATP (a) and 300 μM UTP (b) in electrically driven left atria of SHRs and NWRs. Suramin at 100 μM was present 20 min before adding the nucleotides; baseline atrial contractions were not affected by this compound. Values are expressed as mean±s.e.m. of eight experiments, *P<0.05 difference between NWRs and SHRs, #P<0.05 difference between the inotropic response in presence and absence of antagonist. (one-way ANOVA, Bonferroni post-test).
Effects of the P2 purinoceptor antagonist PPADS on the NIE and PIE response to 300 μM ATP (a) and 300 μM UTP (b) in electrically driven left atria of SHRs and NWRs. PPADS at 10 μM was present 20 min before adding the nucleotides; baseline atrial contractions were not affected by this compound. Values are expressed as mean±s.e.m. of eight experiments, *P<0.05 difference between NWRs and SHRs, #P<0.05 difference between the inotropic response in presence and absence of antagonist. (one-way ANOVA, Bonferroni post-test).
In addition, we tested whether PPADS (10 μM) affected the response to ATP and UTP. PPADS antagonized the PIEs of ATP and UTP (Figures 5a, b). NWRs exhibited 53±1% inhibition of the PIE response to ATP compared with 39±1% in SHRs. For UTP, the PIE response in the presence of suramin was reduced by 57.0±0.8% in NWRs and 43±1% in SHRs. Curiously, the NIE of ATP was potentiated by suramin (Figure 4a) and PPADS in SHRs but not in NWRs (Figure 5a). This was not the case for the UTP NIE (Figures 4b, 5b).
Effect of the L-type calcium channel blocker nifedipine on the biphasic effects of ATP and UTP on atrial contraction
The effects of ATP and UTP on atrial inotropism were investigated in the presence of nifedipine (0.1 μM, preincubated for 20 min). Nifedipine decreased basal contraction by ∼35±2% following incubation for 20 min in both groups (data not shown). The PIEs of 300 μM ATP and UTP were blocked by nifedipine in SHRs and NWRs (Figures 6a, b) and reduced in SHRs by 46±2% compared with 34.0±0.9% in NWRs (P<0.05) (Figure 6a and Table 4). However, the PIE of UTP was more effectively blocked by nifedipine in NWRs (30±1%) compared with SHRs (26.0±0.4%, P<0.05) (Figure 6b and Table 4). In addition, nifedipine did not affect the NIEs ATP or UTP in SHRs or NWRs (Figures 6a, b).
Effects of L-type Ca2+ channel blockers nifedipina on the PIE responses to 300 μM ATP (a) and 300 μM UTP (b) in electrically driven left atria of SHRs and NWRs. Nifedipine 0.1 μM was present 20 min before adding the nucleotides. Values are expressed as mean±s.e.m. of six experiments, *P<0.05 difference between NWRs and SHRs, #P<0.05 difference between the inotropic response in presence and absence of antagonist. (one-way ANOVA, Bonferroni post-test).
Effect of ryanodine on the biphasic effects of ATP and UTP in electrically driven left atria of SHRs and NWRs
At nanomolar concentrations, ryanodine allows the formation of an open channel state of the ryanodine receptor in the endoplasmic reticulum, which triggers the release of calcium and thus results in Ca2+ depletion in this storage organelle.30
Preincubation with ryanodine at 3.0 nM diminished the basal atrial contraction by ∼45.0% in both groups. Moreover, calcium depletion of the endoplasmic reticulum decreased the PIEs of 300 μM ATP and UTP in SHR and NWR atria (Figures 7a, b). The PIE of ATP was slightly inhibited in SHRs (25±2%) compared with NWRs (60±2%, P<0.05) (Figure 7a and Table 4). However, the PIE of UTP was more inhibited by ryanodine in SHRs (59±1%) compared with NWRs (51±1%, P<0.05) (Figure 7b and Table 4).
Effects of ryanodine (sarcoplasmic reticulum calcium depletion triggered) on the PIE responses to 300 μM ATP (a) and 300 μM UTP (b) in electrically driven left atria of SHRs and NWRs. Ryanodine 3.0 nM was present 20 min. before adding the nucleotides. Values are expressed as mean±s.e.m. of 6 experiments, *P<0.05 difference between NWRs and SHRs, #P<0.05 difference between the inotropic response in presence and absence of antagonist. (one-way ANOVA, Bonferroni post-test).
The NIE of ATP was increased 46±4% by ryanodine in SHRs and NWRs (Figure 7a). No changes were observed in the NIE of UTP in either group (Figure 7b).
Effect of the protonophore CCCP on the biphasic effects of ATP and UTP in the left atria of SHRs and NWRs
CCCP is a mitochondrial protonophore that dissipates the proton gradient and causes the depolarization of mitochondria, thus blocking Ca2+ uptake and depleting Ca2+ from the mitochondrial matrix.31 We therefore also explored the effect of CCCP on the effects of ATP and UTP at 300 μM on atrial contractions.
The basal contraction was decreased ∼52±3% by preincubation with CCCP (0.1 μM for 20 min) in SHRs and NWRs. The PIEs of ATP and UTP were significantly decreased by CCCP in both groups (Figure 8). The PIE of ATP was more efficiently inhibited by CCCP in NWRs (57±1%) compared with SHRs (40±1%, P<0.05), as shown in Figure 8a and Table 4. The same was observed for the PIE of UTP (Figure 8b and Table 4).
Effects of CCCP (mitochondrial calcium depletion triggered) on the PIE responses to 300 μM ATP (a) and 300 μM UTP (b) in electrically driven left atria of SHRs and NWRs. CCCP 0.1 μM was present 20 min before adding the nucleotides. Values are expressed as mean±s.e.m. of six experiments, *P<0.05 difference between NWRs and SHRs, #P<0.05 difference between the inotropic response in presence and absence of antagonist. (one-way ANOVA, Bonferroni post-test).
By contrast, the NIE of UTP was augmented by CCCP by 38±3% in SHRs (Figure 8a). No changes were observed in the NIE of UTP in NWRs (Figure 8b).
Discussion
As shown in Table 1, SHRs presented an increased ventricle size and augmented blood pressure, characterizing a hypertensive state. An equivalent situation was described by Okamoto and Aoki26 and Bishop et al.27 In addition, we did not observe any difference in the contractile parameters (Table 2).
Similar to previous reports,14, 15, 16 we demonstrated that electrically driven NWR atria present dual NIE and PIE responses to the purinergic receptor agonists ATP and UTP. Such duality was also observed in SHR atrial preparations, although the responses differed from those of normotensive animals; whereas NIE responses were smaller in SHR atria, the PIE responses were higher than those of NWR atria, and this was true for both ATP and UTP. It appears that in hypertensive animals, the cardiac PIEs of purinoceptor agonists predominate over the NIEs.
The involvement of P1 purinoceptors of the A1 subtype in the blockade of NIE responses by DPCPX was suggested by our results and has previously been shown in normotensive rats.16 PIE responses were likely mediated by P2 purinoceptor subtypes because they were blocked by PPADS and suramin, as previously demonstrated in guinea pig and frog hearts14 and in rat hearts.20, 32 Suramin has been used to characterize the involvement of P2 receptors in the contractile responses of other preparations of vascular tissues,33 mouse vas deferens34 and the guinea pig urinary bladder and taenia coli.35 The extent of the inhibition elicited by PPADS and suramin of the PIEs of ATP and UTP was slightly higher in NWR atria when compared with SHR atria, indicating higher expression of P2 purinoceptors in the latter. This is in agreement with the higher P2 purinoceptor densities previously reported in the tail arteries36 and mesenteric arteries37 of SHRs.
An interesting feature of this study concerns the interaction of P1 and P2 purinoceptors in regulating the NIEs and PIEs induced by ATP and UTP. For instance, the decreased NIEs in the presence of DPCPX were accompanied by enhanced PIEs to ATP in both NWR and SHR atria (Figure 3a). This was not the case for UTP, which elicited a smaller PIE in SHR atria (Figure 3b). Regardless, as previously reported, it appears that adenosine receptor blockade exposes the stimulatory effect of ATP on cardiac contractility.32
The handling of Ca2+ ions by cardiac cells is central in the regulation of heart contractility. Thus, we explored how pharmacological interference in Ca2+ handling affected the NIEs and PIEs induced by ATP and UTP in both normotensive and hypertensive rat atria. The NIEs on NWR atria were little affected by nifedipine, ryanodine or CCCP. However, in SHR atria, ryanodine and CCCP augmented the NIEs, indicating that the endoplasmic reticulum Ca2+ store and mitochondrial Ca2+ handling could be involved in such responses.
Nifedipine more greatly inhibited the PIE of ATP in SHRs. This observation could be explained by activation of P2X receptors by ATP, which increases Ca2+ influx through L-type Ca2+ channels in cardiomyocytes,38 inducing Ca2+ release from the endoplasmic reticulum.39 This may also explain how the ATP-elicited PIE response was depressed in NWR atria compared with SHR atria in ryanodine-treated preparations (Table 4), indicating a greater size of the endoplasmic reticulum Ca2+ store in SHRs. This finding is consistent with our earlier observation that chromaffin cells in adrenal medullary slices from SHRs have a greater endoplasmic reticulum Ca2+ store.11 In this context, a plausible explanation is that the enhanced PIE response to ATP in SHR atria could be associated with increased release of Ca2+ from the endoplasmic reticulum.
Following treatment with CCCP, the PIEs of ATP and UTP were depressed to a lesser extent in SHR atria than in NWR atria (Table 4). This is again compatible with the previous observation that protonophores induce a larger depletion of mitochondrial Ca2+ in SHR adrenal slices compared with those of NWRs.11
From a clinical standpoint, the doses used here are similar to those used in human coronary heart disease.28 This indicates that our results may be useful to for comparison with our expectations for human patients.
Conclusion
In conclusion, the PIEs of ATP and UTP were higher in SHR atria compared with NWR atria. The observed pharmacological interference in Ca2+ handling suggests the existence of an augmented endoplasmic reticulum Ca2+ store and faster mitochondrial Ca2+ cycling in SHR atria than in NWR atria. These data support the hypothesis that dysfunction of purinergic neurotransmission and enhanced sympathetic activity are contributors to the pathogenesis of hypertension.
References
Kaplan NM . Treating prehypertension: a review of the evidence. Curr Hypertens Rep 2008; 10: 326–329 Review.
Anderson EA, Sinkey CA, Lawton WJ, Mark AL . Elevated sympathetic nerve activity in borderline hypertensive humans: evidence from direct intraneural recordings. Hypertension 1989; 14: 177–183.
De Champlain J, Karas M, Toal C, Nadeau R, Larochelle P . Effects of antihypertensive therapies on the sympathetic nervous system. Can J Cardiol 1999; 15 (Suppl A): 8A–14A.
Goldstein Goldstein DS . Plasma catecholamines and essential hypertension: an analytical review. Hypertension 1983; 5: 86–99.
Iriuchijima J . Cardiac output and total peripheral resistance in spontaneously hypertensive rats. Jpn Heart J 1973; 14: 267–272.
Grobecker G, Roizen MF, Weise V, Saavedra JM, Kopin IJ . Letter: sympathoadrenal medullary activity in young, spontaneously hypertensive rats. Nature 1975; 258: 267–268.
Pak CH . Plasma adrenaline and noradrenaline concentrations of the spontaneously hypertensive rat. Jpn Heart J 1981; 22: 987–995.
Hoffman BB . Therapy of hypertension Bruton LL, Lazo JS, Parker KL (eds). Goodman and Gilmańs The Pharmacological Basis of Therapeutics. McGraw-Hill: Madrid, Spain. 2006 pp. 845–868.
Miranda-Ferreira R, de Pascual R, de Diego AM, Caricati-Neto A, Gandía L, Jurkiewicz A, García AG . Single-vesicle catecholamine release has greater quantal content and faster kinetics in chromaffin cells from hypertensive, as compared with normotensive, rats. J Pharmacol Exp Ther 2008; 324: 685–693.
Miranda-Ferreira R, de Pascual R, Caricati-Neto A, Gandía L, Jurkiewicz A, García AG . Role of the endoplasmic reticulum and mitochondria on quantal catecholamine release from chromaffin cells of control and hypertensive rats. J Pharmacol Exp Ther 2009; 329: 231–240.
Miranda-Ferreira R, de Pascual R, Smaili SS, Caricati-Neto A, Gandía L, García AG, Jurkiewicz A . Greater cytosolic and mitochondrial calcium transients in adrenal medullary slices of hypertensive, compared with normotensive rats. Eur J Pharmacol 2010; 636: 126–136.
Erlinge D, Burnstock G . P2 receptors in cardiovascular regulation and disease. Purinergic Signal 2008; 4: 1–20.
Burnstock G . Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 2007; 87: 659–797.
Burnstock G, Meghji P . Distribution of P1- and P2-purinoceptors in the guinea-pig and frog heart. Br J Pharmacol 1981; 73: 879–885.
Fleetwood G, Gordon JL . Purinoceptors in the rat heart. Br J Pharmacol 1987; 90: 219–227.
Froldi G, Pandolfo L, Chinellato A, Ragazzi E, Caparrotta L, Fassina G . Dual effect of ATP and UTP on rat atria: which types of receptors are involved? N-S Arch Pharmacol 1994; 349: 381–386.
Burnstock G, Cocks T, Crowe R . Evidence for purinergic innervation of the anococcygeus muscle. Br J Pharmacol 1978; 64: 13–20.
Kennedy C, Burnstock G . Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur J Pharmacol 1985; 111: 49–56.
O’Connor SE, Dainty IA, Leff P . Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci 1991; 12: 137–141.
Froldi G, Ragazzi E, Caparrotta L . Do ATP and UTP involve cGMP in positive inotropism on rat atria? Comp Biochem Physiol C Toxicol Pharmacol 2001; 128: 265–274.
Manso AM, Encabo A, Ferrer M, Balfagon G, Salaices M, Marin J . Changes of cardiac calcium homeostasis in spontaneously hypertensive rats. J Auton Pharmacol 1999; 19: 123–130.
Fowler MR, Orchard CH, Harrison SM . Cellular distribution of calcium current is unaltered during compensated hypertrophy in the spontaneously hypertensive rat. Pflugers Arch 2007; 453: 463–469.
Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ . Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension 2002; 40: 214–219.
Gandía L, García AG, Morad M . ATP modulation of calcium channels in chromaffin cells. J Physiol 1993; 470: 55–72.
de Pascual R, Miranda-Ferreira R, Galvão KM, Lameu C, Ulrich H, Smaili SS, Jurkiewicz A, García AG, Gandía L . Lower density of L-type and higher density of P/Q-type of calcium channels in chromaffin cells of hypertensive, compared with normotensive rats. Eur J Pharmacol 2013; 706: 25–35.
Okamoto K, Aoki K . Development of a strain of spontaneously hypertensive rats. Jpn Circ J 1963; 27: 282–293.
Bishop SP, Dillon D, Naftilan J, Reynolds R . Surface morphology of isolated cardiac myocytes from hypertrophied hearts of aging spontaneously hypertensive rats. Scan Electron Microsc 1980; Pt 2: 193–199.
Gergs U, Boknik P, Schmitz W, Simm A, Silber RE, Neumann J . A positive inotropic effect of ATP in the human cardiac atrium. Am J Physiol Heart Circ Physiol 2008; 294: H1716–H1723.
Dunn PM, Blakeley AGH . Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol 1988; 93: 243–245.
Meissner G . Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol 1994; 56: 485–508.
Akopova OV, Sagach VF . Relese of Ca2+ from mitochondria after mitochondrial membrane depolarisation. Ukr Biokhim Zh 2005; 77: 62–69.
Mantelli L, Amerini S, Filippi S, Ledda F . Blockade of adenosine receptors unmasks a stimulatory effect of ATP on cardiac contractility. Br J Pharmacol 1993; 109: 1268–1271.
Schlicker E, Urbanek E, Gothert M . ATP, alpha,beta-methylene ATP and suramin as tools for characterization of vascular P2X receptors in the pithed rat. J Auton Pharmacol 1989; 9: 357–366.
Von K I, Bultmann R, Starke K . Effects of suramin and alpha, beta-methylene ATP indicate noradrenaline-ATP co-transmission in the response of the mouse vas deferens to single and low frequency pulses. Naunyn Schmiedebergs Arch Pharmacol 1989; 340: 760–763.
Hoyle CH, Knight GE, Burnstock G . Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol 1990; 99: 617–621.
Vidal M, Hicks PE, Langer SZ . Differential effects of alpha-beta methylene ATP on responses to nerve stimulation in SHR and WKY tail arteries. Naunyn Schmiedebergs Arch Pharmacol 1986; 332: 384–390.
Brock JA, Van Helden DF . Enhanced excitatory junction potentials imesenteric arteries from spontaneously hypertensive rats. Pflugers Arch 1995; 430: 901–908.
Scamps F, Legssyer A, Mayoux E, Vassort G . The mechanism of positive inotropy induced by adenosine triphosphate in rat heart. Circ Res 1990; 67: 1007–1016.
De Young MB, Scarpa A . ATP receptor-induced Ca2+ transients in cardiac myocytes: sources of mobilized Ca2+. Am J Physiol 1999; 257: C750–C758.
Acknowledgements
We thank BSc Gabriel Andrade Alves and Henrique Camara for their help during the preparation of this manuscript and Bruno Palmieri de Souza for providing technical support. CAPES, FAPESP and CNPq (Brazil) provided financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, J., da Silva Jr, E., de Magalhães Galvão, K. et al. Differential regulation of atrial contraction by P1 and P2 purinoceptors in normotensive and spontaneously hypertensive rats. Hypertens Res 37, 210–219 (2014). https://doi.org/10.1038/hr.2013.146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2013.146
Keywords
This article is cited by
-
Quantitative proteomics analysis of the treatment of asthma rats with total flavonoid extract from chamomile
Biotechnology Letters (2020)
-
Reversal of right ventricular remodeling by dichloroacetate is related to inhibition of mitochondria-dependent apoptosis
Hypertension Research (2016)