Abstract
It is unknown whether home blood pressure (BP) variability reduction is associated with target organ damage (TOD) protection independently of home mean BP reduction. We enrolled 310 hypertensive patients whose systolic BP (SBP) at home was over 135 mm Hg. The subjects measured their BP in the morning and evening for 7 days. In addition, we measured urinary albumin excretion (UAE) as a marker of TOD before and after 6 months of candesartan treatment (+thiazidediuretics). At baseline, UAE was associated with average home SBP (r=0.24, P<0.001), the s.d. of home SBP (r=0.15, P=0.011), and the maximum home SBP (r=0.27, P<0.001). During the intervention, significant reductions were found in average home SBP (146±13 vs. 132±12 mm Hg, P<0.001), s.d. of home SBP (12.9±4.8 vs. 11.8±4.4 mm Hg, P<0.001), and maximum home SBP (172.5±18.0 vs. 155.9±17.5 mm Hg, P<0.001). UAE levels were significantly reduced after 6months of therapy compared with baseline (18.9 vs. 12.1 mg g−1 Cre, P<0.001). In multiple regression analysis, the treatment-induced reduction in UAE was significantly associated with that of average home BP (P=0.003) but was not associated with that of s.d. of home SBP or that of maximum home SBP. Home BP variability is not itself an interventional target beyond lowering mean home BP during anti-hypertensive treatment.
Similar content being viewed by others
Introduction
Many studies have focused on the association between blood pressure (BP) variability (BPV) and target organ damage (TOD) or cardiovascular prognosis.1, 2, 3, 4, 5 Studies based on ambulatory BP monitoring have provided evidence that BPV over 24 h was associated with the severity of organ damage and the rate of cardiovascular events.6, 7 Recently, in conventional office BP measurements, increased visit-to-visit variability, calculated as the s.d. of the mean of repeated recordings and maximum systolic BP (SBP) over a certain period, has been reported to indicate increased cardiovascular risk.5
We have reported that not only office BPV but also day-to-day BPV evaluated by home BP (HBP) monitoring (HBPM) is associated with TOD (that is, cardiac hypertrophy and carotid artery remodeling) independently of average office or HBP levels.8, 9 Moreover, Kikuya et al.4 reported that the increased day-to-day BPV, calculated as the s.d. of HBP, was associated with cardiovascular mortality in a community-dwelling population. However, it is not clear whether home BPV itself, beyond the mean HBP level, can contribute directly to the development of TOD. The treatment-induced change in TODs assessed by cardiovascular biomarkers, such as the level of urinary albumin excretion (UAE), is associated with prognosis;10, 11, 12 therefore, precise assessment of changes in cardiovascular biomarkers may help identify patients in need of more aggressive clinical management.
The aim of this study is to assess whether or not the reduction in HBPvariability, as evaluated by s.d., is associated with UAE independently of home mean BP reduction upon intervention with anti-hypertensive treatment.
Methods
Our study represented a post-hoc subgroup analysis focusing on home BP data obtained byassessing home BPV in the Japan Morning Surge−Target Organ Protection (J-TOP) study, which was originally designed to investigate the impact of the dosing time of candesartan on cardiorenal damage in hypertensive patients. The detailed protocol was described previously.13 Briefly, the J-TOP study included hypertensive outpatients with elevated morning or evening SBP (⩾135 mm Hg) as assessed by HBPM, and both BP thresholds and the target in this study focused on morning or evening HBP levels. The study was conducted from 29 July 2005 to 31 July 2008, by eight doctors at eight institutions (three primary carefacilities, three hospital-based outpatient clinics and two specialized university hospitals) in Japan. The Ethics Committee of the Internal Review Board at Jichi Medical University (Tochigi, Japan) approved the protocol. Written informed consent was obtained from each subject enrolled in this study. The study protocol was registered on a clinical trials registration site, UMIN (University Hospital Medical Information Network) Clinical Trials Registry (UMIN-CTR): #UMIN000001139).
Study subjects and study design
We enrolled 454 hypertensive patients whose morning or evening HBP, as determined by self-measured systolic HBP, was over 135mm Hg and who had either been under stable antihypertensive treatment for the past 3 months or who were currently unmedicated. We did not recruit patients with arrhythmia, a history of congestive heart failure, a recent history (within 6 months) of coronary artery disease, stroke, aortic dissection, or peripheral artery disease, dementia, the presence of a malignancy or chronic inflammatory disease, or bilateral renal arterial stenosis. Nor did we recruit patients with a contraindication for candesartan.
The enrolled subjects were randomized into a bedtime-dosing group and an awakening-dosing group. Patients were initially given candesartan 4 mg per day shortly after randomization if they were not already taking an angiotensin receptor blocker (ARB) at the time of enrollment. Patients taking another ARB at the time of enrollment were changed to candesartan 8 mg per day shortly after the randomization of the timing of dosing. This randomization was carried out at an independent research center. The dose of candesartan was increased to 8 mg per day and then to 12 mg per day at follow-up visits, which were performed at monthly intervals until morning SBP or evening SBP remained no higher than 135 mm Hg. If morning or evening SBP was still ⩾135 mm Hg after 12 mg per day of candesartan, then a diuretic (indapamide, trichlormethiazide or hydrochlorothiazide) was added in both bedtime-dosing and awakening-dosing groups except in patients who were receiving a diuretic at initial enrollment. The follow-up study period was 6 months. Other baseline anti-hypertensive medications were not changed throughout the study period (including timing of administration).
Blood pressure measurement
We asked patients to measure their morning and evening home BP in a sitting position. BP was measured using a validated cuffoscillometric device (HEM-5001; Omron Healthcare, Kyoto, Japan) according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension.14 This self-measured HBPM automatically made three measurements at 15-s intervals on each occasion. All recorded BP parameters were stored in its memory; the BP and pulse rate data were transferred to a PC for storage at the beginning of the study and at the end of the 6-month follow-up period. We computed the average BP and within-subject s.d. as home BPV using morning and evening BP data for 7 days before visiting (42 readings in total) at both the beginning of the study and the end of the 6-month follow-up. In addition, we selected the within-subject maximum BP levelfrom the 42 readings. Clinic BP was measured at the clinic using the same device, and was calculated as the average of three consecutive measurements.
Urinary examination
UAE was measured using a turbidimetric immunoassay (SRL Inc., Tokyo, Japan) and expressed as the urinary albumin/creatinine ratio (mg g−1·Cr). Urine creatinine was measured by enzymatic assay. The intra-/inter-coefficients of variation were 1.30%/1.85% for UAE. Urine samples were collected in the morning with the subjects fasting, at the beginning of the study, and at the end of 6-month follow-up.
Statistical analysis
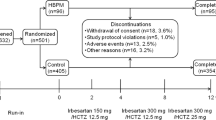
Of the 454 patients enrolled in the present study, we analyzed 310 whose HBP data were successfully transferred and stored to a PC at both the beginning of the study and the end of the 6-month follow-up (Figure 1). Data were expressed as means (±s.d.) or percentages. Because the distribution of UAE was highly skewed, it was log-transformed before statistical analysis. The paired t-test was used to test temporal changes in the means. Spearman correlation coefficients were used to measure the relationships between BP parameters and UAE at baseline, and were also used to measure the relationships between the change in UAE and the change in HBP parameters from the beginning of the study to the 6-month follow-up. Multiple linear regression analyses were performed to estimate and test the independent effects of the relationship between BP parameters and UAE at baseline and changes in HBP parameters on changes in UAE. In the initial model (Model 1 in Table 3), the associations between average SBP change and UAE change were assessed after adjustment for age, sex, body mass index, smoking, drinking, history of cardiovascular disease, the use of any antihypertensive drug at baseline, dose timing of candesartan (+diuretic), and urinary albumin/creatinine ratio at baseline. Extended models included maximum SBP change (Model 2 in Table 3), s.d. of SBP change, and maximum SBP changes (Model 3 in Table 3). Differences with a P-value <0.05 (two-tailed) were considered statistically significant throughout the study. The computer software package SPSS version 11.0J (SPSS, Chicago, IL, USA) was used for the analyses.
Results
Table 1 shows the baseline characteristics of all patients. The awakening-dosing group and bedtime-dosing group consisted of 156 and 154 patients, respectively. A diuretic was added for 169 patients (54.5%) because their systolic HBP did not reach the target level of 135 mm Hg with 12 mg candesartan.
Table 2 shows the average, maximum and s.d. of home SBP and DBP, as well as the changes in those variables after 6 months of follow-up. All BP parameters were significantly reduced after the follow-up period. Compared with baseline, UAE was significantly reduced at the end of the study (median (25%, 75%): 18.9 (9.1, 48.5) vs. 12.1 (7.0, 23.3) mg g−1 Cre, P<0.001).
The relationship between BP parameters and UAE at baseline
Figure 2 shows the association between BP parameters and UAE at baseline. Average SBP, s.d. of SBP and maximum SBP were positively correlated with UAE. In multiple linear regression analysis, both average SBP (Standardized β=0.24, P<0.001) and s.d. of SBP (standardized β=0.12, P=0.035) were associated with UAE adjusted for age, gender, body mass index, smoking, drinking, hyperlipidemia, diabetes, history of cardiovascular disease and use of an antihypertensive drug. On the other hand, DBP parameters were not associated with UAE (average DBP: r=−0.06, P=0.3, s.d. of DBP: r=0.05, P=0.423, maximum DBP: r=0.04, P=0.526).
The relationship between the change in BP parameters and UAE
As shown in Figure 3, in univariate analysis, the reduction in UAE was associated with reductions in average, maximum and s.d. of SBP. These associations were found also with DBP parameters (average DBP: r=0.26, P<0.001, s.d. of DBP: r=0.17, P=0.002, maximum DBP: r=0.23, P<0.001). Next, we divided the patients into those receiving a diuretic (n=169) and those without diuretic add-on therapy (n=141). In the latter group, the reduction in UAE was associated with the reduction in all SBP and DBP parameters. In the group receiving a diuretic, the reduction in UAE was associated with the reduction in average SBP and average DBP, as well as with maximum SBP and DBP. However, no association was found in s.d. between SBP and DBP (data not shown).
We performed multiple regression analysis in three models to investigate whether the reduction in home BPV is associated with an improvement in UAE independently of the reduction in mean HBP level. In all models, the reduction in mean SBP was independently associated with the reduction in UAE (Table 3).
The reduction in average SBP was associated with that of the maximum SBP (r=0.80, P<0.001) and the s.d. (r=0.21, P<0.001). The reduction in average DBP was associated with that of maximum DBP (r=0.58, P<0.001) but was not associated with that of s.d. of DBP (r=0.05, P=0.395).
Discussion
The main findings of the present study are as follows: (i) in the cross-sectional analysis at baseline, not only average HBP levels but also day-to-day BPV, assessed by the s.d. of home SBP, were associated with UAE independently of other covariates; (ii) although intervention with antihypertensive treatment significantly reduced both average HBP level and day-to-day BPV, the reduction in the latter was not associated with the improvement in UAE. Our results may indicate that, in the clinical management of hypertension, day-to-day BPV itself cannot be an interventional target beyond lowering mean HBP level.
This result might challenge previous findings. Kikuya et al.4 have reported that day-to-day BPV, assessed by the calculation of s.d. in home BP, was associated with cardiovascular events in a Japanese general population. That study found a treated hypertension prevalence of 30% and did not include the evaluation of TOD in the analysis model, which investigated the association between day-to-day BPV and cardiovascular events. Nor did that study describe the hypertension treatment conditions during follow-up. Therefore, it is not clear whether reducing BPV would lead to better outcomes. Recently, we have also reported that day-to-day BPV, assessed by the calculation of s.d. in HBP during 14 days, was associated with UAE, left ventricular mass index, and carotid intima-media thickness independently of mean HBP level among untreated hypertensive subjects.9 In that study, in the goodness-of-fit of the model, maximum home SBP did not improve information about the progression of UAE.9 Although this might mean that the association between home BPV and UAE was relatively weak, this finding might differ depending on the characteristics of the population under study. From these previous reports, however, we cannot infer whether or not home BPV reduction can lead to cardiovascular protection independently of home mean BP reduction. As excess BPV was recognized in subjects with advanced arterial stiffness or target-organ damage,8, 9 we do not understand whether excess BPV is merely a marker of advanced target-organ damages or is a treatable risk factor that leads to cardiovascular protection.
In the present study we demonstrated for the first time that to predict the treatment-induced reduction in UAE during anti-hypertensive treatment, the treatment-induced reduction in BPV adds no significant value to that in average home SBP levels. However, it might be too early to conclude from this study that the goal of reducing BPV to improve cardiovascular events is small compared with the target of average BP level. Rothwell et al.15 reported that visit-to-visit clinic SBP variability during a 5-year follow-up in patients receiving calcium-channel blockers was lower than that in patients receiving beta blockers to explain the disparity in observed effects on the risk of stroke. The follow-up period in our study, 6 months, might be not enough to allow exploration of the impact of BPV reduction on changes in organ damage.
Every other BPV tested has been induced to increase the prognostic ability of BP, over and above the information provided by average BP level. Although day-to-day BPV is an index of BPV during the medium term, BPV within a 24-h period assessed by ABPM and visit-to-visit variability assessed by clinic BP are indices of BP instability during the short and long terms, respectively.16 There is no consensus on a single definition of the method to evaluate day-to-day BPV. Kikuya et al.4 evaluated measuring BP once every morning and evening to calculate day-to-day variability, while Johansson et al.17 evaluated measuring BP twice every morning and evening. In this study, we measured BP in triplicate on each occasion. Thus, future studies should determine the optimal number of HBP measurements needed to obtain reliable and valid estimates of the day-to-day BPV.BPV, estimated as the s.d. of BP obtained by noninvasive ABPM, has been suggested as a risk factor for hypertension-related TOD.3, 7 In addition, BP instability, such as morning BP surge and morning-evening BP difference, were assessed by ambulatory and HBP measurements, respectively.18, 19 All have been shown to give prognostic information, independentof that provided by average BP values. The intervention for these different components of BPV may reflect different mechanisms and have different clinical implications for cardiovascular events.
The present study has some limitations. First, it was performed by post-hoc analysis. The number of subjects included in this analysis might appear to be relatively small for a study focusing on the effects of day-to-day BPV on organ damage. Second, this study does not show the association between day-to-day BPV and any other TOD or cardiovascular prognosis. The lowering of albuminuria has been independently associated with cardiovascular protection.10, 20 However, some papers have recently reported that the change in albuminuria did not correlate with a hard outcome, that is cardiovascular events.21, 22 Thus, whether albuminuria with a range of normal to microalbuminuria is a surrogate marker for cardiovascular events remains a subject of ongoing debate. In addition, Ushigome et al.23 reported that type 2 diabetic patients with macroalbuminuria had a higher day-to-day BPV than those without. In patients with macroalbuminuria, protection against kidney damage might be better related to BPV than to absolute BP levels. Finally, to perform this analysis, we excluded some of the patients enrolled in this study, because their HBP measurements were incomplete. Including the patients whom we excluded might lead to a better result for HBP reduction and improvement in home BPV during treatment than we report here. HBP monitoring has been recognized and widely adopted. It is clear that the reduction in average HBP level with hypertensive treatment leads to an improvement in cardiovascular damage. Although day-to-day BPV is an index of cardiovascular damage, it might not itself be an interventional target beyond lowering mean HBP during anti-hypertensive treatment. Our finding shows that it is important for practicing physicians to first control average HBP level without concern that day-to-day variability might increase the risk of cardiovascular damage.
References
Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y . Prognostic significance of blood pressure and heart rate variabilities: The Ohasama study. Hypertension 2000; 36: 901–906.
Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O'Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH . Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens 2003; 21: 2251–2257.
Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, Ferrario M, Mancia G . Blood pressure variability and organ damage in a general population: results from the PAMELA study. Hypertension 2002; 39: 710–714.
Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y . Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: The Ohasama study. Hypertension 2008; 52: 1045–1050.
Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR . Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010; 375: 895–905.
Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Telera MP, Pede S, Gattobigio R, Porcellati C . Adverse prognostic value of a blunted circadian rhythm of heart rate in essential hypertension. J Hypertens 1998; 16: 1335–1343.
Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R . Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 2007; 50: 325–332.
Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K . Visit-to-visit blood pressure variations: new independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens 2011; 5: 184–192.
Matsui Y, Ishikawa J, Eguchi K, Shibasaki S, Shimada K, Kario K . Maximum value of home blood pressure: a novel indicator of target organ damage in hypertension. Hypertension 2011; 57: 1087–1093.
Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlof B, Devereux RB, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wan Y . Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45: 198–202.
Araki S, Haneda M, Koya D, Hidaka H, Sugimoto T, Isono M, Isshiki K, Chin-Kanasaki M, Uzu T, Kashiwagi A . Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 2007; 56: 1727–1730.
Maisel AS . Cardiovascular and renal surrogate markers in the clinical management of hypertension. Cardiovasc Drugs Ther 2009; 23: 317–326.
Kario K, Hoshide S, Shimizu M, Yano Y, Eguchi K, Ishikawa J, Ishikawa S, Shimada K . Effect of dosing time of angiotensin ii receptor blockade titrated by self-measured blood pressure recordings on cardiorenal protection in hypertensives: The Japan morning surge-target organ protection (J-TOP) study. J Hypertens 2010; 28: 1574–1583.
Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H . The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2009). Hypertens Res 2009; 32: 3–107.
Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Poulter NR, Sever PS . Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010; 9: 469–480.
Stergiou GS, Parati G . How to best assess blood pressure?: the ongoing debate on the clinical value of blood pressure average and variability. Hypertension 2011; 57: 1041–1042.
Johansson JK, Niiranen TJ, Puukka PJ, Jula AM . Factors affecting the variability of home-measured blood pressure and heart rate: The Finn-home study. J Hypertens 2010; 28: 1836–1845.
Matsui Y, Eguchi K, Shibasaki S, Shimizu M, Ishikawa J, Shimada K, Kario K . Association between the morning-evening difference in home blood pressure and cardiac damage in untreated hypertensive patients. J Hypertens 2009; 27: 712–720.
Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K . Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107: 1401–1406.
Holtkamp FA, de Zeeuw D, de Graeff PA, Laverman GD, Berl T, Remuzzi G, Packham D, Lewis JB, Parving HH, Lambers Heerspink HJ . Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J 2011; 32: 1493–1499.
Lattanzio MR, Weir MR . Have we fallen off target with concerns surrounding dual raas blockade? Kidney Int 2010; 78: 539–545.
Bakris GL, Sarafidis PA, Weir MR, Dahlof B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V, Chiang YT, Weber MA . Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet 2010; 375: 1173–1181.
Ushigome E, Fukui M, Hamaguchi M, Senmaru T, Sakabe K, Tanaka M, Yamazaki M, Hasegawa G, Nakamura N . The coefficient variation of home blood pressure is a novel factor associated with macroalbuminuria in type 2 diabetes mellitus. Hypertens Res 2011; 34: 1271–1275.
Acknowledgements
SH analyzed the data from this study and was responsible for the writing of this paper. YY and KK were the advisers for the conception and design of this study, and assisted in conducting the statistical analyses. MS, KE, and JI recruited the study patients. This study was financially supported in part by a grant from the Japan Heart Foundation, Tokyo (JHF-05-155). The study protocol was registered on a clinical trials registration site, UMIN (University Hospital Medical Information Network) Clinical Trials Registry (UMIN-CTR): #UMIN000001139].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hoshide, S., Yano, Y., Shimizu, M. et al. Is home blood pressure variability itself an interventional target beyond lowering mean home blood pressure during anti-hypertensive treatment?. Hypertens Res 35, 862–866 (2012). https://doi.org/10.1038/hr.2012.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2012.46
Keywords
This article is cited by
-
Annual reports on hypertension research 2020
Hypertension Research (2022)
-
Maximum home blood pressure readings are associated with left atrial diameter in essential hypertensives
Journal of Human Hypertension (2018)
-
Blood Pressure Variability: Assessment, Predictive Value, and Potential as a Therapeutic Target
Current Hypertension Reports (2015)
-
Blood pressure variability assessed by home measurements: a systematic review
Hypertension Research (2014)