Abstract
The Millennium Genome Project for Hypertension was started in 2000 to identify genetic variants conferring susceptibility to hypertension, with the aim of furthering the understanding of the pathogenesis of this condition and realizing genome-based personalized medical care. Two different approaches were launched, genome-wide association analysis using single-nucleotide polymorphisms (SNPs) and microsatellite markers, and systematic candidate gene analysis, under the hypothesis that common variants have an important role in the etiology of common diseases. These multilateral approaches identified ATP2B1 as a gene responsible for hypertension in not only Japanese but also Caucasians. The high blood pressure susceptibility conferred by certain alleles of ATP2B1 has been widely replicated in various populations. Ex vivo mRNA expression analysis in umbilical artery smooth muscle cells indicated that reduced expression of this gene associated with the risk allele may be an underlying mechanism relating the ATP2B1 variant to hypertension. However, the effect size of a SNP was too small to clarify the entire picture of the genetic basis of hypertension. Further, dense genome analysis with accurate phenotype data may be required.
Similar content being viewed by others
Introduction
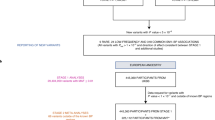
In 2000, a number of national cooperative projects were started in Japan under the banner of ‘Millennium Projects’. These projects aimed to achieve bold technological innovation in three areas of vital importance to Japan: information utilization, aging society and the environment. Among them, genome research in five fields of genetics, namely disease genes, human genome variation, the rice genome, bioinformatics, and development/differentiation/regeneration, was collectively termed the ‘Millennium Genome Project (MGP)’. This project was launched to shed light on some of the unsolved challenges of the aging society by gaining an understanding of disease pathogenesis and by establishing genome-based personalized medical care (Figure 1).1 In the Disease Genes division, the five most frequent diseases in developed countries that have a harmful impact on lifelong health and quality-of-life were selected, namely essential hypertension, diabetes, cancer, asthma and Alzheimer's disease. Among the study groups for these five target diseases, the group for hypertension consists of 16 researchers whose focus is the genetic aspects of essential hypertension (Figure 2). These researchers were selected from among scientists with established achievements in genetics and/or epidemiology of hypertension. The aim of this review is to summarize the results of gene hunting conducted by the study group for hypertension.
Study overview
Two antithetical but complementary approaches were adopted to identify common variants associated with individual blood pressure susceptibility, a genetic statistics-based genome-wide approach and a knowledge-based candidate gene approach (Figure 3). A multi-stage genome-wide association study (GWAS) using approximately 80 000 gene-associated single-nucleotide polymorphisms (SNPs) was performed as a five study group initiative as the flagship study of the Disease Genes division. The Japanese-oriented SNPs (JSNPs) analyzed in this GWAS2, 3 and the high-throughput low-cost genotyping system based on the Invader technology were both developed by the Human Genome Variation division.
The study group for hypertension undertook a second GWAS using approximately 20 000 microsatellite (MS) markers with an average spacing of 146 kb. Although many studies have used commercially available MS marker sets consisting of <1000 markers to narrow down candidate gene loci, a Japanese research group has successfully identified 30 000 MS marker sets by systemic whole-genome screening and developed a high-throughput genotyping system based on the DNA-pooling method.4
The candidate gene approach was concomitantly designed and performed by each of the study groups. The study group for hypertension performed a systemic multiple candidate gene analysis, with candidate gene selection made from among genes related to signal transduction pathways, including receptors, soluble carrier proteins, binding proteins, channels, enzymes and G-proteins that were possibly related to blood pressure regulation.
GWAS using SNP markers
To overcome financial and technological limitations, mostly owing to a lack of DNA chip technology, the Disease Genes division launched a coordinated two-stage-screening approach (Figure 4).5 The theoretical basis of this exploratory test scheme has been reported elsewhere.6 In the first stage, a total of 940 samples from 188 patients with one of the five target diseases were simultaneously genotyped, and the association analysis was performed using the case samples of the other four study groups as a pseudo control. Allele frequency data for 752 individuals from a general Japanese population established by the JSNP project were also used as a second control. Both this exploratory analysis as well as the second-stage analysis used cases of men with very severe hypertension gathered by the nationwide MGP collaboration. Among the 80 795 SNPs initially genotyped, the 2676 top-hit SNPs that showed an odds ratio (OR) >1.4 with a P-value <0.015 in at least one test comparison of allele frequency or genotype distribution were analyzed in the next stage analysis. A total of 75 SNPs that showed positive associations in the second-stage analysis, namely a P-value <0.01 for genotype distribution and <0.05 for allele frequency on comparison of 940 cases (188 first-stage cases and 752 second-stage cases) and 752 controls, were further analyzed in a second panel of 619 hypertensive subjects and 1406 normotensive controls. After the third-stage analysis, which was the study group’s own extensive analysis, six SNPs located in four genes, ADD2, EYA2, KIAA0789 and M6PR, were finally explored.
Identification of the ADD2 gene as a candidate locus for BP regulation is particularly noteworthy, because β-adducin, which is encoded by the ADD2 gene, has been suggested to have a role in a physiological pathway of BP regulation.7 Adducin is a heterodimeric cytoskeleton protein composed of α-, β- and γ-subunits, and is highly conserved across species. Point mutations on the Add1 and Add2 genes account for the 50% BP difference between Milan hypertensive and normotensive strains via the modulation of Na+–K+ activity.8, 9 In humans, association of the ADD1 G460W polymorphism (rs4961) with hypertension has been widely investigated in various populations7 under the hypothesis that the ADD1 W allele accelerates Na+–K+ co-transport,10 which in turn results in increased renal sodium reabsorption. As for the ADD2 gene, several articles have reported that this may confer susceptibility in association analyses.11, 12 A linkage analysis also reported that the 2p13 region in ADD2 was also a hypertension-related locus.13 However, a recent large-scale GWAS in the European population,14 as well as our study in East Asians,15 found no SNPs of genome-wide significance near the ADD2 gene, or the other three identified genes, namely EYA2, KIAA0789 (renamed to WSCD2) and M6PR. Statistical power of previous GWAS was not enough to detect SNPs with smaller effect sizes. As larger GWAS samples can detect larger numbers of common susceptibility variants with smaller effects, increasing sample size may provide more convincing evidence.
GWAS using MS markers
A total of 385 hypertensive patients and 385 normotensive control subjects were analyzed in the three-stage screening using 18 977 MS markers (Figure 3).16 A portionof the hypertensive cases analyzed in the first and second screenings were shared with the SNP-based GWAS. To bring down the cost and technical burden of genotyping 1000 MS markers, individual DNA samples of each stage were pooled within cases and controls. The pooled DNA was then amplified using the 18 977 MS markers, and the PCR products were genotyped by a standard protocol using capillary DNA sequencers. This DNA-pooling method enabled us to obtain the allele frequencies of MS markers in pooled Japanese individuals by measuring the height of multiple peaks. An association analysis was performed by comparing the height of peaks between the case and controls. P-values <0.05 were considered significant in all stage analyses to avoid potential false negatives.
After the three-stage analysis, 54 markers, which showed P-values <0.05 in either of the 2 × 2 (allelic) or 2 × m (genotype, m=number of alleles) analysis, were initially identified as potential susceptible loci for hypertension. These markers were again genotyped in all 770 individual samples (not pooled) used in the first- to third-stage analyses (385 cases and 385 controls), and finally 19 loci were identified. Among these 19 loci, three chromosomal locations, 6q27, 2p25.1 and 2p11.1-q12.3, overlapped with a region identified in other linkage17, 18 or admixture mapping studies.19 Although these previous studies did not permit sufficient narrowing down of the region to allow a candidate gene to be identified, our dense mapping did allow the former two markers to be dropped into the SMOC2 and LPIN1 gene regions, respectively. Although the SMOC2 gene encodes a matricellular protein that promotes matrix assembly and can stimulate endothelial cell proliferation and migration, an association between this gene and hypertension has not been suggested. Further, LPIN1 is recognized as a candidate gene for human lipodystrophy, which is characterized by a loss of body fat, fatty liver, hypertriglyceridemia and insulin resistance. Although one study reported replication data which suggested that one of the 12 tagged SNPs in the LPIN1 gene region was a susceptibility locus for hypertension in Chinese men,20 another study failed to replicate the associations.21
Systematic candidate gene screening
Candidate gene analysis is the classic approach to find susceptible genotypes. Previous studies selected several candidate genes on the basis of known biochemical or physiologic components related to BP regulation. The results of previous studies which investigated a single or only a few arbitrarily selected genes have not been consistent, and positive findings have rarely been replicated. As hypertension is a polygenic disease, alleles at many uninvestigated loci would have contributed to the ultimate disease trait. We therefore conducted a systematic candidate gene screening by evaluating 307 genes selected from those related to signal transduction, binding proteins, channels, enzymes and G-proteins that were possibly related to BP regulation.22, 23
The first screening was conducted using a case–control panel with 758 severe young-onset hypertensive patients and 726 strictly normotensive elderly persons enrolled from four independent regions throughout Japan (Table 1). One SNP per one gene, preferably located in the promoter region or exons with the highest minor allele frequency in the Japanese population, was selected based on the information published in the JSNP database (http://snp.ims.u-tokyo.ac.jp). Of the 307 SNPs analyzed, 38 showed P-values <0.05 in either analysis comparing allele frequency or genotype distribution, including a dominant or recessive model. To further identify susceptible SNPs, replication genotyping was performed using an independent nested population of 1929 cases and 1993 controls chosen from the approximately 14 000 cohort samples (Table 1). This cohort panel consist of six independent population- or company employee-based genetic epidemiological cohorts established by several of the members of the study group for hypertension. Results repeatedly showed the association of ATP2B1 rs2070759 with hypertension (OR=1.18, P=4.0 × 10−4). The subsequent dense SNP analysis around the 150 kb region of the ATP2B1 gene identified another significant SNP at the promoter region (rs11105378: OR=1.31, P=4.1 × 10−11), and the BP susceptibility of this SNP was replicated in the whole cohort sample analysis. Further, a trans-ethnic analysis using the Global BPgen data set,24 a large-scale GWAS samples of European descent, confirmed that the ATP2B1 gene was a susceptibility locus for hypertension (OR=1.13, P=5.9 × 10−4).
The per-allele effect size of rs11105378 for systolic BP trait was 1.33 mm Hg (P=1.5 × 10−8) in the Japanese cohort sample. Meta-analysis with samples of the Global BPgen (0.59 mm Hg, P=0.001) and the CHARGE25 (1.31 mm Hg, P=9.1 × 10−11) consortia allowed more precise estimation, at 1.02 mm Hg for systolic BP (P=1.4 × 10−18) and 0.54 mm Hg for diastolic BP (P=1.6 × 10−13). Further, combined analysis with other susceptible SNPs identified by recent GWAS in samples of European origin, namely FGF5 rs1458038, CYP17A1 rs1004467 and CSK rs1378942, showed a stepwise association with BP traits and hypertension risk. Individuals carrying seven or eight risk genotypes had higher systolic/diastolic BP (136.2/81.5 mm Hg), in contrast to the lower BP of individuals with only two or less-risk genotypes (125.3/57.5 mm Hg) (Figure 5). The OR of the high-risk group (OR=1.43, P=1.0 × 10−4) was 2.27 compared with the lowest risk group(OR=0.63, P=0.020).
Adjusted OR for hypertension and systolic blood pressure by the number of risk genotypes. Number of risk genotypes was calculated by the following four SNPs: ATP2B1 rs1105378, FGF5 rs1458038, CYP17A1, rs1004467 and CSK rs1378942. Age, age,2 sex, BMI and cohort variable-adjusted OR and systolic BP (line graph) is shown in the panel. Number of subjects in each group is represented as a bar graph.
In the SNP-based MGP-GWAS, two SNPs located in ATP2B1 gene region, namely rs2070758 (TT/TG/GG=153/34/1) and rs2070759 (AA/AC/CC=49/102/35), were genotyped. However, in the association analysis with752 pseudo control samples, the P-value did not reach statistical significance (allele frequency; P=0.460 and P=0.341, respectively). MS markers located 2.4 kb upstream of the ATP2B1 gene also did not show a positive association with hypertension. The most plausible reason for the failure to identify ATP2B1 in the MGP-GWAS may be insufficient statistical power owing to a limited number of subjects. The susceptibility conferred by the ATP2B1 gene is therefore clear, given the negative results of the MGP-GWAS.
Replication results of the BP susceptibility of ATP2B1
GWAS in people of European descent25 and Koreans26 also reported that the ATP2B1 gene variants are the most significant determinants of BP traits and hypertension. The same results were also observed in another Japanese population.27 A meta-analysis of the East-Asian GWAS data28 further supported that the association was strongest for ATP2B1, except for the ALDH2 rs671 genotype, which determines alcohol intolerance in East-Asians. A very recently published world-wide study for BP genetics29 based on 270 000 persons in various populations, including our MGP data sets, provided conclusive evidence on this issue.
Mechanisms of the association between ATP2B1 and BP
The ATP2B1 (so-called PMCA1) gene encodes the plasma membrane calcium ATPase isoform 1, which removes bivalent calcium ions from eukaryotic cells against very large concentration gradients and has a critical role in intracellular calcium homeostasis. Although the pathophysiological implications of ATP2B1 gene products in the development of hypertension are uncertain, we obtained an important clue for the mechanism from an ex vivo expression analysis of ATP2B1 mRNA. ATP2B1 mRNA expression in umbilical artery smooth muscle cells differed among rs11105738 genotypes, and cells carrying the risk allele showed significantly lower levels of mRNA.23
In mammals, calcium ATPase isoforms are encoded by at least four separate genes (ATP2B1–ATP2B4).30 Although we examined the possible association of ATP2B4 gene polymorphisms with hypertension, the most promising of the four isoforms, we found no significant SNPs around the region, suggesting that the association of the plasma membrane Ca2+ pump with BP regulation is isoform-specific.
We recently reported31 that the trimetric intracellular cation A (TRIC-A) channel knockout mouse showed markedly higher BP, and that several SNPs locating around the TRIC-A gene were significantly associated with hypertension in humans. TRIC channels are intracellular monovalent cation channels, which are postulated to mediate counter-ion movements facilitating physiological Ca2+ release from internal stores. It is particularly interesting that the two genes, which were identified by the independent two antithetical approaches of non-hypothesis statistic-based analysis (ATP2B1) and hypothesis-based analysis (TRIC-A), are molecules that play important roles in Ca2+ homeostasis (Figure 6). Dense SNP analysis or re-sequencing of other molecules relating to Ca2+ transport may further elucidate genetic factors for BP regulation.
Perspectives
Hypertension is one of the most prevalent complex genetic disorders, for which the genetic heritability has been estimated at up to 60%. Owing to its large impact on a number of cardiovascular diseases, hypertension is a major contributor to the global health burden. Identification of genetic variants associated with BP regulation is important in two aspects, recognition of genetically high-risk populations for effective prevention and early diagnosis and treatment, and elucidation of the molecular pathogenesis to identify target molecules for diagnosis, treatment and prevention. A series of genetic studies in the MGP for hypertension and recent GWAS in various populations identified several genes responsible for BP regulation. However, the effect size of each implicated SNP was small (<1 mm Hg), and the combination of all identified loci accounted for <10% of overall BP variation. As GWAS are firstly designed to identify common genetic variants for multifactorial diseases and have limited ability to detect less common or rare variants, missing heritability may be traced by analyzing the uncommon variants using a million chip based on the 1000G project data (www.1000genomes.org) or a next generation sequencer. BP is a phenotype that widely fluctuates as a result of non-genetic factors. Accurate BP measurement by ambulatory monitoring or home-measurement may also be indispensable for unlocking the genetic basis of hypertension.
References
Yoshida T, Yoshimura K . Outline of disease gene hunting approaches in the Millennium Genome Project of Japan. Proc Japan Acad 2003; 79: 34–50.
Hirakawa M, Tanaka T, Hashimoto Y, Kuroda M, Takagi T, Nakamura Y . JSNP: a database of common gene variations in the Japanese population. Nucleic Acids Res 2002; 30: 158–162.
Haga H, Yamada R, Ohnishi Y, Nakamura Y, Tanaka T . Gene-based SNP discovery as part of the Japanese Millennium Genome Project: identification of 190,562 genetic variations in the human genome. Single-nucleotide polymorphism. J Hum Genet 2002; 47: 605–610.
Tamiya G, Shinya M, Imanishi T, Ikuta T, Makino S, Okamoto K, Furugaki K, Matsumoto T, Mano S, Ando S, Nozaki Y, Yukawa W, Nakashige R, Yamaguchi D, Ishibashi H, Yonekura M, Nakami Y, Takayama S, Endo T, Saruwatari T, Yagura M, Yoshikawa Y, Fujimoto K, Oka A, Chiku S, Linsen SE, Giphart MJ, Kulski JK, Fukazawa T, Hashimoto H, Kimura M, Hoshina Y, Suzuki Y, Hotta T, Mochida J, Minezaki T, Komai K, Shiozawa S, Taniguchi A, Yamanaka H, Kamatani N, Gojobori T, Bahram S, Inoko H . Whole genome association study of rheumatoid arthritis using 27 039 microsatellites. Hum Mol Genet 2005; 14: 2305–2321.
Kato N, Miyata T, Tabara Y, Katsuya T, Yanai K, Hanada H, Kamide K, Nakura J, Kohara K, Takeuchi F, Mano H, Yasunami M, Kimura A, Kita Y, Ueshima H, Nakayama T, Soma M, Hata A, Fujioka A, Kawano Y, Nakao K, Sekine A, Yoshida T, Nakamura Y, Saruta T, Ogihara T, Sugano S, Miki T, Tomoike H . High-density association study and nomination of susceptibility genes for hypertension in the Japanese National Project. Hum Mol Genet 2008; 17: 617–627.
Sato Y, Suganami H, Hamada C, Yoshimura I, Yoshida T, Yoshimura K . Designing a multistage, SNP-based, genome screen for common diseases. J Hum Genet 2004; 49: 669–676.
Bianchi G, Ferrari P, Staessen JA . Adducin polymorphism: detection and impact on hypertension and related disorders. Hypertension 2005; 45: 331–340.
Bianchi G, Tripodi G, Casari G, Salardi S, Barber BR, Garcia R, Leoni P, Torielli L, Cusi D, Ferrandi M . Two point mutations within the adducin genes are involved in blood pressure variation. Proc Natl Acad Sci U S A. 1994; 91: 3999–4003.
Zagato L, Modica R, Florio M, Torielli L, Bihoreau MT, Bianchi G, Tripodi G . Genetic mapping of blood pressure quantitative trait loci in Milan hypertensive rats. Hypertension 2000; 36: 734–739.
Glorioso N, Filigheddu F, Cusi D, Troffa C, Conti M, Natalizio M, Argiolas G, Barlassina C, Bianchi G . alpha-Adducin 460Trp allele is associated with erythrocyte Na transport rate in North Sardinian primary hypertensives. Hypertension 2002; 39: 357–362.
Tikhonoff V, Kuznetsova T, Stolarz K, Bianchi G, Casiglia E, Kawecka-Jaszcz K, Nikitin Y, Tizzone L, Wang JG, Staessen JA European Project on Genes in Hypertension Investigators Blood pressure phenotypes in relation to the beta-adducin C1797T polymorphism in the European Project on Genes in Hypertension (EPOGH). Blood Press Monit 2003; 8: 151–154.
Wang JG, Staessen JA, Barlassina C, Fagard R, Kuznetsova T, Struijker-Boudier HA, Zagato L, Citterio L, Messaggio E, Bianchi G . Association between hypertension and variation in the alpha- and beta-adducin genes in a white population. Kidney Int 2002; 62: 2152–2159.
Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, Turner ST, Weder AB, Boerwinkle E . Family Blood Pressure Program. Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension 2004; 43: 477–482.
International Consortium for Blood Pressure Genome-Wide Association Studies, E Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478: 103–109.
Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen CH, Zhang Y, Yamamoto K, Katsuya T, Yokota M, Kim YJ, Ong RT, Nabika T, Gu D, Chang LC, Kokubo Y, Huang W, Ohnaka K, Yamori Y, Nakashima E, Jaquish CE, Lee JY, Seielstad M, Isono M, Hixson JE, Chen YT, Miki T, Zhou X, Sugiyama T, Jeon JP, Liu JJ, Takayanagi R, Kim SS, Aung T, Sung YJ, Zhang X, Wong TY, Han BG, Kobayashi S, Ogihara T, Zhu D, Iwai N, Wu JY, Teo YY, Tai ES, Cho YS, He J . Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet 2011; 43: 531–538.
Yatsu K, Mizuki N, Hirawa N, Oka A, Itoh N, Yamane T, Ogawa M, Shiwa T, Tabara Y, Ohno S, Soma M, Hata A, Nakao K, Ueshima H, Ogihara T, Tomoike H, Miki T, Kimura A, Mano S, Kulski JK, Umemura S, Inoko H . High-resolution mapping for essential hypertension using microsatellite markers. Hypertension 2007; 49: 446–452.
Caulfield M, Munroe P, Pembroke J, Samani N, Dominiczak A, Brown M, Benjamin N, Webster J, Ratcliffe P, O'Shea S, Papp J, Taylor E, Dobson R, Knight J, Newhouse S, Hooper J, Lee W, Brain N, Clayton D, Lathrop GM, Farrall M, Connell J . MRC British Genetics of Hypertension Study.Genome-wide mapping of human loci for essential hypertension. Lancet 2003; 361: 2118–2123.
Rice T, Rankinen T, Province MA, Chagnon YC, Pérusse L, Borecki IB, Bouchard C . Rao DC.Genome-wide linkage analysis of systolic and diastolic blood pressure: the Québec Family Study. Circulation 2000; 102: 1956–1963.
Zhu X, Luke A, Cooper RS, Quertermous T, Hanis C, Mosley T, Gu CC, Tang H, Rao DC, Risch N, Weder A . Admixture mapping for hypertension loci with genome-scan markers. Nat Genet 2005; 37: 177–181.
Ong KL, Leung RY, Wong LY, Cherny SS, Sham PC, Lam TH, Lam KS, Cheung BM . Association of a polymorphism in the lipin 1 gene with systolic blood pressure in men. Am J Hypertens 2008; 21: 539–545.
Burgdorf KS, Sandholt CH, Sparsø T, Andersen G, Witte DR, Jørgensen T, Sandbaek A, Lauritzen T, Sørensen TI, Madsbad S, Hansen T, Pedersen O . Studies of association between LPIN1 variants and common metabolic phenotypes among 17,538 Danes. Eur J Endocrinol 2010; 163: 81–87.
Kohara K, Tabara Y, Nakura J, Imai Y, Ohkubo T, Hata A, Soma M, Nakayama T, Umemura S, Hirawa N, Ueshima H, Kita Y, Ogihara T, Katsuya T, Takahashi N, Tokunaga K, Miki T . Identification of hypertension-susceptibility genes and pathways by a systemic multiple candidate gene approach: the millennium genome project for hypertension. Hypertens Res 2008; 31: 203–212.
Tabara Y, Kohara K, Kita Y, Hirawa N, Katsuya T, Ohkubo T, Hiura Y, Tajima A, Morisaki T, Miyata T, Nakayama T, Takashima N, Nakura J, Kawamoto R, Takahashi N, Hata A, Soma M, Imai Y, Kokubo Y, Okamura T, Tomoike H, Iwai N, Ogihara T, Inoue I, Tokunaga K, Johnson T, Caulfield M, Munroe P Global Blood Pressure Genetics Consortium. Umemura S, Ueshima H, Miki T . Common variants in the ATP2B1 gene are associated with susceptibility to hypertension: the Japanese Millennium Genome Project. Hypertension. 2010; 56: 973–980.
Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Döring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvänen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dörr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Völker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Völzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB Wellcome Trust Case Control Consortium Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009; 41: 666–676.
Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM . Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41: 677–687.
Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Lee JK, Oh B, Kim HL . A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet 2009; 41: 527–534.
Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, Nabika T, Fujioka A, Ohnaka K, Asano H, Yamori Y, Yamaguchi S, Kobayashi S, Takayanagi R, Ogihara T, Kato N . Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation 2010; 121: 2302–2309.
Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen CH, Zhang Y, Yamamoto K, Katsuya T, Yokota M, Kim YJ, Ong RT, Nabika T, Gu D, Chang LC, Kokubo Y, Huang W, Ohnaka K, Yamori Y, Nakashima E, Jaquish CE, Lee JY, Seielstad M, Isono M, Hixson JE, Chen YT, Miki T, Zhou X, Sugiyama T, Jeon JP, Liu JJ, Takayanagi R, Kim SS, Aung T, Sung YJ, Zhang X, Wong TY, Han BG, Kobayashi S, Ogihara T, Zhu D, Iwai N, Wu JY, Teo YY, Tai ES, Cho YS, He J . Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet 2011; 43: 531–538.
International Consortium for Blood Pressure Genome-Wide Association Studies Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478: 103–109.
Carafoli E . The Ca2+ pump of the plasma membrane. J Biol Chem 1992; 267: 2115–2118.
Yamazaki D, Tabara Y, Kita S, Hanada H, Komazaki S, Naitou D, Mishima A, Nishi M, Yamamura H, Yamamoto S, Kakizawa S, Miyachi H, Yamamoto S, Miyata T, Kawano Y, Kamide K, Ogihara T, Hata A, Umemura S, Soma M, Takahashi N, Imaizumi Y, Miki T, Iwamoto T, Takeshima H . TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab 2011; 14: 231–241.
Acknowledgements
We greatly appreciate Drs Patricia Munroe, Toby Johnson and Mark Caulfield in Queen Mary University of London for their kind cooperation with this work. This work was supported by Grants for Scientific Research (Priority Areas ‘Medical Genome Science (Millennium Genome Project)’ and ‘Applied Genomics’, Leading Project for Personalized Medicine, and Scientific Research 20390185, 21390099, 19659163, 16790336, 12204008, 15790293, 16590433, 17790381, 17790381, 18390192, 18590265, 18590587, 18590811, 19590929, 19650188, 19790423, 17390186, 20390184, 21390223, 21390099) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan; Grants-in-Aid (H15-Longevity-005, H17-longevity-003, H16-kenko-001, H18-longevity (kokusai), H11-longevity-020, H17-Kenkou-007, H17-pharmaco-common-003, H18-Junkankitou[Seishuu]-Ippan-012 and H20-Junkankitou[Seishuu]-Ippan-009, 013) from the Ministry of Health, Labor and Welfare, Health and Labor Sciences Research Grants, Japan; a Science and Technology Incubation Program in Advanced Regions, Japan Science and Technology Agency; The Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO); Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS) fellows (16.54041, 18.54042, 19.7152, 20.7198, 20.7477 and 20.54043); the Japan Atherosclerosis Prevention Fund; the Uehara Memorial Foundation; the Takeda Medical Research Foundation; National Cardiovascular Research Grants; Biomedical Innovation Grants; and the Japan Research Foundation for Clinical Pharmacology.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The following authors have been named as inventors on a patent application by Ehime University, Shiga University of Medical Science and Yokohama City University in work related to this study: Yasuharu Tabara, Katsuhiko Kohara, Yoshikuni Kita, Nobuhito Hirawa, Jun Nakura, Satoshi Umemura, Hirotsugu Ueshima and Tetsuro Miki.
Appendix
Appendix
The study group of the Millennium Genome Project for Hypertension; authors of the representative three papers5,16,23 from the study group were alphabetically listed
- Akihiro Fujioka:
-
Amagasaki Health Medical Foundation, Amagasaki, Japan
- Hironori Hanada:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Akira Hata:
-
Graduate School of Medicine, Chiba University, Chiba, Japan
- Nobuhito Hirawa:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Yumiko Hiura:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Yutaka Imai:
-
Tohoku University Graduate School of Pharmaceutical Science and Medicine
- Hidetoshi Inoko:
-
Tokai University School of Medicine, Isehara, Japan
- Itsuro Inoue:
-
School of Medicine, Tokai University, Isehara, Japan
- Norihiko Itoh:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Naoharu Iwai:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Jerzy K. Kulski:
-
Murdoch University, Murdoch, Western Australia, Austaralia.
- Kei Kamide:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Norihiro Kato:
-
National Center for Global Health and Medicine, Tokyo, Japan
- Tomohiro Katsuya:
-
Osaka University Graduate School of Medicine, Suita, Japan
- Ryuichi Kawamoto:
-
Ehime University Graduate School of Medicine, Toon, Japan
- Yuhei Kawano:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Akinori Kimura:
-
School of Biomedical Science, Tokyo Medical and Dental University, Tokyo, Japan,
- Yoshikuni Kita:
-
Shiga University of Medical Science, Otsu, Japan
- Katsuhiko Kohara:
-
Ehime University Graduate School of Medicine, Toon, Japan
- Yoshihiro Kokubo:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Hiroyuki Mano:
-
Jichi Medical University, Shimotsuke-shi, Japan
- Shuhei Mano:
-
Nagoya City University, Nagoya, Japan
- Tetsuro Miki:
-
Ehime University Graduate School of Medicine, Toon, Japan
- Toshiyuki Miyata:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Nobuhisa Mizuki:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Takayuki Morisaki:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Yasuyuki Nakamura:
-
Kyoto Women's University, Kyoto, Japan
- Kazuwa Nakao:
-
Kyoto University Graduate School of Medicine, Kyoto, Japan,
- Tomohiro Nakayama:
-
Nihon University School of Medicine, Tokyo, Japan
- Jun Nakura:
-
Ehime University Graduate School of Medicine, Toon, Japan
- Momoko Ogawa:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Toshio Ogihara:
-
Osaka University Graduate School of Medicine, Suita, Japan,
- Takayoshi Ohkubo:
-
Tohoku University Graduate School of Pharmaceutical Science and Medicine
- Shigeaki Ohno:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Akira Oka:
-
Tokai University School of Medicine, Isehara, Japan
- Tomonori Okamura:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Takao Saruta:
-
School of Medicine, Keio University, Tokyo, Japan
- Akihiro Sekine:
-
Institute of Physical and Chemical Research, Tokyo, Japan
- Tadashi Shiwa:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Masayoshi Soma:
-
Nihon University School of Medicine, Tokyo, Japan
- Sumio Sugano:
-
Graduate School of Frontier Sciences, University of Tokyo, Tokyo, Japan
- Yasuharu Tabara:
-
Ehime University Graduate School of Medicine, Toon, Japan
- Atsushi Tajima:
-
School of Medicine, Tokai University, Isehara, Japan
- Norio Takahashi:
-
Radiation Effects Research Foundation, Hiroshima, Japan
- Naoyuki Takashima:
-
Shiga University of Medical Science, Otsu, Japan
- Fumihiko Takeuchi:
-
National Center for Global Health and Medicine, Tokyo, Japan
- Katsushi Tokunaga:
-
Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
- Hitonobu Tomoike:
-
National Cerebral and Cardiovascular Center, Suita, Japan
- Hirotsugu Ueshima:
-
Shiga University of Medical Science, Otsu, Japan
- Satoshi Umemura:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Takahiro Yamane:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Kazuyuki Yanai:
-
National Center for Global Health and Medicine, Tokyo, Japan
- Michio Yasunami:
-
School of Biomedical Science, Tokyo Medical and Dental University, Tokyo, Japan,
- Keisuke Yatsu:
-
Yokohama City University Graduate School of Medicine, Yokohama, Japan
- Teruhiko Yoshida:
-
National Cancer Center, Tokyo, Japan
Rights and permissions
About this article
Cite this article
Tabara, Y., Kohara, K., Miki, T. et al. Hunting for genes for hypertension: the Millennium Genome Project for Hypertension. Hypertens Res 35, 567–573 (2012). https://doi.org/10.1038/hr.2012.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2012.41