Abstract
The aim of this study was to explore the role of circulating endothelial progenitor cells (EPCs) and endothelial apoptotic microparticles in hypertensive patients with and without electrocardiographic left ventricular hypertrophy (LVH). Flow cytometry was used to assess endothelial cell apoptosis and circulating EPC level by quantification of circulating EPC markers (defined as CD34+CD133+, CD34+KDR+) and endothelial apoptotic microparticles (defined as CD31+/annexin V+) in peripheral blood samples. The LVH was defined by ECG with the Cornell voltage criteria. In total, 128 hypertensive patients (83 men and 45 women, aged 59±14 years) were enrolled in this study, in which 107 patients (84%) showed no electrocardiographic evidence of LVH, and 21 patients (16%) fulfilled the LVH criteria by ECG. There were no significant differences in basic characteristics between the two groups, but hypertensive patients with LVH had a higher urine albumin excretion rate than those without LVH (P=0.027). Furthermore, hypertensive patients with LVH were shown to have decreased circulating EPC numbers (all P<0.05) and adhesive function compared with those without LVH (LVH vs. no LVH: 14±6 vs. 30±6 cells per high-power field, P<0.001). Increased numbers of endothelial apoptotic microparticles were noted in hypertensive patients with LVH (4.2±4.9 vs. 2.4±3.4%, P=0.115), although the difference was not significant. This study showed that essential hypertensive patients with electrocardiographic LVH evidence have decreased circulating EPC numbers and adhesive function compared with those without LVH. These findings may explain the pathogenetic processes that link hypertensive LVH and endothelial injury in cardiovascular disease.
Similar content being viewed by others
Introduction
Left ventricular hypertrophy (LVH) is a hypertensive target organ damage strongly predictive of future cardiovascular morbidity and mortality.1, 2, 3 Direct cardiac effects of LVH include a higher risk of developing congestive heart failure, increased risk of arrhythmic events, reduced coronary flow reserve and promotion of myocardial ischemic episodes.4 Moreover, clinical evidence indicates that impaired endothelium-dependent vasodilation is recognized in hypertensive patients with cardiac hypertrophy.3, 5 However, the pathophysiological mechanisms underlying the evolution from LVH to endothelial dysfunction and cardiovascular event development remain to be determined.
The normal endothelium has a pivotal role in the local regulation of vascular tone and relaxation by producing and releasing contracting and relaxing factors.6 Endothelial dysfunction is considered the first step of vascular remodeling and takes place during the development of atherosclerosis in the systemic circulation.7 Recent insight suggests that the injured endothelial monolayer is regenerated by circulating bone marrow-derived endothelial progenitor cells (EPCs),8 and levels of circulating EPCs reflect endothelial repair capacity.9 A reduced number of circulating EPCs independently predicts atherosclerotic disease progression and future cardiovascular events,10 thus supporting an important role for endogenous vascular repair by EPCs. Such repairs modulate the clinical course of coronary artery disease. Furthermore, endothelial microparticles carry membrane proteins and phospholipids of parent cells (for example, CD31 when derived from endothelial cells) and can also be derived from microparticles from leukocytes, erythrocytes or platelets.11 The number of apoptotic microparticles has been shown to be elevated in conditions of endothelial cell damage,12 and increases in patients with acute coronary syndrome, coronary artery disease and hypercholesterolemia.13, 14, 15 However, no previous study has mentioned the roles of circulating EPCs and endothelial apoptotic microparticles in hypertensive patients with or without LVH. In this study, we tested the hypothesis that decreased circulating EPC levels and increased numbers of endothelial apoptotic microparticles might be associated with electrocardiographic evidence of LVH in hypertensive patients.
Methods
Study participants
A total of 128 subjects with essential hypertension were enrolled in the study between June 2008 and July 2009. Hypertension was defined as having a systolic blood pressure ⩾140 mm Hg, a diastolic blood pressure ⩾90 mm Hg or the use of antihypertensive drugs. In hypertensive patients, ECG evidence of LVH was defined by Cornell voltage criteria. S wave in V3 plus R wave in aVL >24 mm in men and S wave in V3 plus R wave in aVL >20 mm in women were considered ECG evidence of LVH.16 None of the patients had a history or clinical evidence of angina, myocardial infarction, congestive heart failure, valvular heart disease, peripheral vascular disease, renal failure, inflammatory disease, coagulopathy or any disease predisposing patients to vasculitis or Raynaud’s phenomenon. Causes of secondary hypertension were excluded by appropriate investigations, including measurement of plasma renin activity and aldosterone, Doppler studies of the renal arteries and/or renal scintigraphy. Medical history, including cardiovascular risk factors, previous and present cardiovascular events, as well as current drug treatment, was obtained during a personal interview and from medical files. All patients provided their informed consent, and the study was approved by the local research ethics committee.
Laboratory investigations
The blood pressure of all subjects was measured, and their body mass index was calculated by dividing the weight of the patient in kilograms by the square of the height in meters. Blood pressure values were based on the average of three different measurements taken after 15-min resting periods. Fasting blood glucose, uric acid, creatinine, glycated hemoglobin, total cholesterol, high-density lipoprotein cholesterol and triglyceride levels in blood samples drawn after a 12-h fast were measured with the standard method.
After 8 h of overnight fasting, all subjects had a venous blood sample taken for measurement of high-sensitivity C-reactive protein (hsCRP). Plasma hsCRP levels were assessed using latex-enhanced immunonephelometric assay (Dade Behring, Marburg, Germany).17 The intra-assay and inter-assay variation coefficients were not more than 4 and 8%, respectively. Estimated creatinine clearance rate was calculated by the Cockcroft–Gault formula.18 Overnight urine samples were obtained for measurement of the albumin excretion rate.
Assay of circulating EPCs
A volume of 100 μl peripheral blood was incubated for 15 min in the dark, with monoclonal antibodies against human kinase insert domain-conjugating receptor (KDR; R&D, Minneapolis, MN, USA), followed by phycoerythrin-conjugated secondary antibody, with the fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies against human CD45 (Becton Dickinson, Franklin Lakes, NJ, USA), with the phycoerythrin-conjugated monoclonal antibody against human CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany) or with FITC- or phycoerythrin-conjugated monoclonal antibodies against human CD34 (Serotec, Raleigh, NC, USA) and KDR (Sigma, St Louis, MO, USA).18 Isotype-identical antibodies served as controls (Becton Dickinson). After incubation, cells were lysed, washed with phosphate-buffered saline and fixed in 2% paraformaldehyde before analysis. Each analysis included 100 000 events. The numbers of circulating EPCs were gated with monocytes and defined as CD34+KDR+CD45low and CD34+AC133+CD45low, respectively. To assess the reproducibility of EPC measurements, circulating EPCs were measured from two separate blood samples in 10 subjects, and there was a strong correlation between the two measurements (r=0.90, P<0.001).
Human EPC colony-forming assay
Isolated mononuclear cells were resuspended in growth medium (EndoCult liquid medium, StemCell Technologies, Vancouver, British Columbia, Canada), and 5 × 106 mononuclear cells were preplated in a fibronectin-coated six-well plate in duplicate.19 After 48 h, the non-adherent cells were collected by pipetting the medium in each well up and down three times, and 1 × 106 cells were replated onto a fibronectin-coated 24-well plate. On day 5 of the assay, the number of colony-forming units per well for each sample was counted. All colonies were counted manually in a minimum of three wells by two independent observers.
Assay of circulating CD31+/annexin V+ microparticles
Plasma derived from 10 ml citrate-buffered blood was immediately centrifuged at 13 000g for 2 min to generate platelet-poor plasma. A volume of 50 μl of platelet-poor plasma was incubated with 4 μl of phycoerythrin-conjugated monoclonal antibody against CD31 (Becton Dickinson), followed by incubation with FITC-conjugated annexin V, according to the manufacturer’s instructions.18 IgG-FITC (Pharmingen, San Jose, CA, USA) served as a negative control. Fluorescence-activated cell sorting analysis was performed immediately after staining, using a fluorescence-activated cell sorting Calibur instrument (Becton Dickinson). CD31+/annexin V+ microparticles were defined as particles positively labeled for CD31 and annexin V (CD31+/annexin V+). To reduce the number of microparticles derived from non-endothelial cells, which may occasionally show low expression of CD31, only CD31bright microparticles were selected.
Fibronectin adhesion assay of EPCs
Early EPCs (day 7) from 10 subjects (5 hypertensive subjects with LVH, 5 without LVH) were washed with phosphate-buffered saline and gently detached with 0.5 mmol l−1 EDTA in phosphate-buffered saline. After centrifugation and resuspension in basal medium with 5% fetal bovine serum, identical cells were placed on fibronectin-coated six-well plates and incubated for 30 min at 37 °C. Gentle washing with phosphate-buffered saline was performed three times after 30 min adhesion, and adherent cells were counted by independent blinded investigators.20 As shown in Figure 1, phenotyping of endothelial characteristics of adherent cells by indirect immunostaining was performed with FITC-labeled lectin from Ulex europaeus (Sigma). Briefly, the adherent cells were fixed in 2% paraformaldehyde and incubated with 10 μg ml−1 FITC-labeled lectin from Ulex europaeus (Sigma), as previously described.20
Statistical analysis
Data were expressed as the means±s.d. for numeric variables and as the number (percent) for categorical variables. Comparisons of continuous variables between groups were performed by Student's t-test. Subgroup comparisons of categorical variables were assessed by a χ2 or Fisher's exact test. To examine the effects of various factors on electrocardiographic LVH, the following factors were considered simultaneously as independent variables for multivariate logistic regression analysis: age, sex, systolic blood pressure, EPC marker (CD34+/KDR+), apoptotic microparticles (CD31+/annexin V+) and urinary albumin excretion rate. Data were analyzed using SPSS software (version 15, SPSS, Chicago, IL, USA). A P-value of <0.05 was considered to indicate statistical significance.
Results
Patient characteristics
The mean age of 128 hypertensive patients (45 females, 35%) was 59±14 years. The patients were classified into two groups, including 107 patients (84%) without ECG evidence of LVH and 21 patients (16%) with ECG evidence of LVH. The baseline characteristics of all patients are presented in Table 1. No significant differences were noted between the two groups, including age, sex, body mass index, glomerular filtration rate, serum creatinine, systolic and diastolic blood pressure, pulse pressure, smoking status, serum blood glucose and serum lipid profiles. However, hypertensive patients with LVH have higher rates of urine albumin excretion than those without LVH (P=0.027, Table 1). There were no significant differences with regard to the use of anti-hypertension medication in the two groups, including angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, calcium-channel blockers, diuretics and statins. Moreover, the plasma concentrations of hsCRP (LVH vs. no LVH: 0.29±0.19 vs. 0.38±0.46 mg l−1, P=0.38) revealed no significant differences in hypertensive patients with or without electrocardiographic LVH.
Circulating EPCs, colony-forming units and endothelial apoptotic microparticles
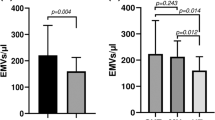
As shown in Table 2, hypertensive patients with electrocardiographic LVH had significantly decreased numbers of circulating EPCs (LVH vs. no LVH, CD34+/KDR+: 0.319±0.333 vs. 0.710±0.729%, P<0.001; CD34+/AC133+: 0.362±0.301 vs. 0.663±0.646%, P=0.002) and decreased EPC colony-forming units (LVH vs. no LVH, 26±12 vs. 34±12 colony-forming units per well, P=0.018) compared with those without electrocardiographic LVH. Hypertensive patients with LVH had higher levels of circulating CD31+/annexin V+ apoptotic microparticles compared with those without ECG evidence of LVH (LVH vs. no LVH subjects, CD31+/annexin V+: 4.2±4.9 vs. 2.4±3.4%, P=0.115), but the statistic was not significant. In addition, significantly attenuated EPC fibronectin adhesion function was found in hypertensive patients with LVH compared with those without LVH (LVH vs. no LVH: 14±6 vs. 30±6 cells per high-power field, P<0.001; Figure 1).
Independent correlates of electrocardiographic LVH
To identify the independent predictors for ECG evidence of LVH, multivariate logistic regression analysis was performed. After adjustment for age, sex, systolic blood pressure, EPC marker (CD34+/KDR+), apoptotic microparticles (CD31+/annexin V+) and urine albumin excretion rate (μg min−1), only EPC marker (CD34+/KDR+, P=0.028) was independently associated with electrocardiographic LVH in hypertensive patients, as shown in Table 3.
Discussion
To the best of our knowledge, this is the first study to show that levels and adhesive function of circulating EPCs in hypertensive patients with electrocardiographic LVH decreased compared with those without ECG evidence of LVH. These findings indicate that attenuated endothelial repair capacity may contribute to atherosclerotic disease progression and enhanced cardiovascular events in hypertensive patients with LVH.
LVH is hypertensive target organ damage strongly predictive of future cardiovascular morbidity and mortality.1, 2, 3 Direct cardiac effects of LVH include an enhanced risk of developing congestive heart failure, an increased risk of sudden cardiac death21 and promotion of myocardial ischemic episodes.4 This cardiovascular risk can be reduced by effective treatment that induces regression of left ventricular mass.22 Moreover, clinical study indicates that impaired endothelium-dependent vasodilation is recognized in hypertensive patients with LVH.3, 5 However, the pathophysiological mechanisms underlying the evolution from hypertensive LVH to endothelial dysfunction and cardiovascular event development remain to be determined.
Convincing evidence suggests that the integrity and functional activity of the endothelial monolayer has an important role in atherogenesis.23 In humans, extensive endothelial cell damage caused by cardiovascular risk factors can result in endothelial cell apoptosis with subsequent loss of integrity of the endothelium. The extent of endothelial injury may represent a balance between the magnitude of injury and the capacity for repair, and predicts cardiovascular event rates. The traditional view suggests that endothelial cell repair is exclusively mediated by the adjacent endothelial cells. However, a series of basic and clinical studies prompted by the discovery of bone marrow-derived EPCs have provided new insights into these processes and indicate that circulating EPCs have a pivotal role in endothelial cell regeneration.24 EPCs are bone marrow-derived cells that enter the systemic circulation to replace defective or injured mature endothelial cells.25 Reduced levels of circulating EPCs independently predict atherosclerotic disease progression and future cardiovascular events,10 thus supporting an important role for endogenous vascular repair by EPCs to modulate the clinical course of coronary artery disease. The balance between endothelium injury and repair is critical for the maintenance of vascular homeostasis. Therefore, enhancement of circulating EPC level and its functional capacity by lifestyle modification or pharmacological strategies may be of clinical importance and therapeutic potential. In this study, we showed for the first time that hypertensive patients with ECG evidence of LVH had decreased circulating EPC levels and attenuated EPC adhesive function, implying attenuated endothelial repair capacity in hypertensive patients with cardiac hypertrophy. This is in agreement with previous studies showing that left ventricular mass in hypertensive patients is inversely related to endothelium-dependent vasodilation in hypertensive patients.5 The association between cardiac hypertrophy and endothelial dysfunction resulting from impaired vascular repair capacity may contribute to the pathogenesis of the high incidence of vascular events that is well documented in hypertensive patients with LVH.
Moreover, the plasma concentrations of hsCRP did not differ significantly between hypertensive patients with or without electrocardiographic LVH evidence. This finding is consistent with previous reports showing that soluble tumor necrosis factor receptor-1, but not hsCRP or interleukin-6, is independently associated with increased LV mass.26 Although hsCRP is associated with measures of the burden of atherosclerosis, such as carotid artery intima-media thickness, hsCRP might be a more specific predictor of plaque vulnerability and hence future cardiovascular events, rather than of the extent of atherosclerosis in many epidemiological studies.
Endothelial microparticles carry membrane proteins and phospholipids of parent cells (for example, CD31 when derived from endothelial cells) and can be derived from microparticles from leukocytes, erythrocytes or platelets.27 Apoptotic microparticles have been shown to be elevated in conditions of endothelial cell damage28 and to increase in patients with acute coronary syndrome, coronary artery disease and hypercholesterolemia.14, 15, 29 Although the prognostic potential of circulating microparticles is still in its infancy, the different studies mentioned above clearly demonstrate that their detection and quantification are interesting and potentially valuable tools to appreciate cardiovascular risk in asymptomatic patients. An interesting finding in the current study is that hypertensive patients with LVH had enhanced levels of circulating endothelial apoptotic microparticles compared with those without ECG evidence of LVH, although the difference did not reach statistical significance. These findings imply that attenuated vascular repair capacity displays early signs before extensive endothelial damage occurs in hypertensive LVH patients. Therefore, intensive and multifactorial interventions are recommended for hypertensive patients with ECG evidence of cardiac hypertrophy.
Study limitations
Some limitations should be mentioned in this study. First, the study population is relatively small and further studies are needed to verify the result. Second, electrocardiographic diagnosis of LVH used in the study generally has a problem of frequent false positivity and should be considered a limitation. Third, it is well know that hypertension causes endothelial dysfunction and lowers circulating EPC levels. Patients with LVH in this study were shown to have a higher rate of excretion of urine albumin, which is a marker of endothelial dysfunction. Therefore, LVH may just be a bystander rather than the cause or effect of decreased circulating EPC levels.
Conclusions
This study demonstrated for the first time that essential hypertensive patients with electrocardiographic evidence of LVH have decreased circulating EPC numbers and adhesive function compared with those without LVH evidence. These findings may explain the pathogenetic processes that link hypertensive LVH and endothelial injury in cardiovascular disease.
References
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH . Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114: 345–352.
Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, Ambrosio G . Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001; 104: 2039–2044.
Salles GF, Fiszman R, Cardoso CR, Muxfeldt ES . Relation of left ventricular hypertrophy with systemic inflammation and endothelial damage in resistant hypertension. Hypertension 2007; 50: 723–728.
Kahan T . The importance of left ventricular hypertrophy in human hypertension. J Hypertens 1998; 16: 23–29.
Perticone F, Maio R, Ceravolo R, Cosco C, Cloro C, Mattioli PL . Relationship between left ventricular mass and endothelium-dependent vasodilation in never-treated hypertensive patients. Circulation 1999; 99: 1991–1996.
Vane JR, Anggård EE, Botting RM . Regulatory functions of the vascular endothelium. N Engl J Med 1990; 323: 27–36.
Bonetti PO, Lerman LO, Lerman A . Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 2003; 23: 168–175.
Werner N, Priller J, Laufs U, Endres M, Böhm M, Dirnagl U, Nickenig G . Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol 2002; 22: 1567–1572.
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T . Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348: 593–600.
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G . Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353: 999–1007.
Horstman LL, Jy W, Jimenez JJ, Ahn YS . Endothelial microparticles as markers of endothelial dysfunction. Front Biosci 2004; 9: 1118–1135.
Jimenez JJ, Jy W, Mauro LM, Horstman LL, Soderland C, Ahn YS . Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activation. Br J Haematol 2003; 123: 896–902.
Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A . Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000; 101: 841–843.
Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G . Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2006; 26: 112–116.
Pirro M, Schillaci G, Paltriccia R, Bagaglia F, Menecali C, Mannarino MR, Capanni M, Velardi A, Mannarino E . Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006; 26: 2530–2535.
Molloy TJ, Okin PM, Devereux RB, Kligfield P . Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage duration. J Am Coll Cardiol 1992; 20: 1180–1186.
Huang PH, Lu TM, Wu TC, Lin FY, Chen YH, Chen JW, Lin SJ . Usefulness of combined high-sensitive C-reactive protein and N-terminal-probrain natriuretic peptide for predicting cardiovascular events in patients with suspected coronary artery disease. Coron Artery Dis 2008; 19: 187–193.
Huang PH, Huang SS, Chen YH, Lin CP, Chiang KH, Chen JS . Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J Hypertens 2010; 28: 1655–1665.
Huang PH, Chen YH, Tsai HY, Chen JS, Wu TC, Lin FY, Sata M, Chen JW, Lin SJ . Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler Thromb Vasc Biol 2010; 30: 869–877.
Huang PH, Chen YH, Chen YL, Wu TC, Chen JW, Lin SJ . Vascular endothelial function and circulating endothelial progenitor cells in patients with cardiac syndrome X. Heart 2007; 93: 1064–1070.
Haider AW, Larson MG, Benjamin EJ, Levy D . Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32: 1454–1459.
Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B . Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292: 2343–2349.
Fuster V, Badimon L, Badimon JJ, Chesebro JH . The pathogenesis of coronary artery disease and the acute coronary syndrome. N Engl J Med 1992; 326: 310–318.
Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA . Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003; 108: 457–463.
Urbich C, Dimmeler S . Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 2004; 95: 343–353.
Zhou Z, Peng J, Wang CJ, Li D, Li TT, Hu CP, Chen XP, Li YJ . Accelerated senescence of endothelial progenitor cells in hypertension is related to the reduction of calcitonin gene-related peptide. J Hypertens 2010; 28: 931–939.
Takei Y, Di Tullio MR, Homma S, Boden-Albala B, Rundek T, Sacco RL, Berry G, Liu R, Jin Z, Eguchi K, Elkind MS . Soluble tumor necrosis factor receptor 1 level is associated with left ventricular hypertrophy: the northern Manhattan study. Am J Hypertens 2009; 22: 763–769.
Horstman LL, Jy W, Jimenez JJ, Ahn YS . Endothelial microparticles as markers of endothelial dysfunction. Front Biosci 2004; 9: 1118–1135.
Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A . Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000; 101: 841–843.
Acknowledgements
This study was partly supported by research grants NSC 96-2320-B-039-042, NSC 97-2314-B-075-039 and NSC 98-2314-B-075-035 from the National Science Council; VGH-97DHA0100127, VGH-ER-2-97DHA0100664 and V98B1-003 from Taipei Veterans General Hospital; CI 96-16, CI 97-14 from the Yen Tjing Ling Medical Foundation, Taipei, Taiwan, and a grant from the Ministry of Education, Aim for the Top University Plan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, CW., Huang, PH., Huang, SS. et al. Decreased circulating endothelial progenitor cell levels and function in essential hypertensive patients with electrocardiographic left ventricular hypertrophy. Hypertens Res 34, 999–1003 (2011). https://doi.org/10.1038/hr.2011.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.68
Keywords
This article is cited by
-
Circulating endothelial and angiogenic cells predict hippocampal volume as a function of HIV status
Journal of NeuroVirology (2023)
-
Extracellular Vesicles in Essential Hypertension: Hidden Messengers
Current Hypertension Reports (2020)
-
Association of circulating progenitor cells with angiotensin II in newly diagnosed hypertensive patients
Journal of Human Hypertension (2018)
-
Involvement of NADPH oxidases and non-muscle myosin light chain in senescence of endothelial progenitor cells in hyperlipidemia
Naunyn-Schmiedeberg's Archives of Pharmacology (2016)
-
Early Outgrowth Pro-Angiogenic Cell Number and Function Do Not Correlate with Left Ventricular Structure and Function in Conventional Hemodialysis Patients: A Cross-Sectional Study
Canadian Journal of Kidney Health and Disease (2015)