Abstract
Ω-3 polyunsaturated fatty acid deficiency, particularly during the prenatal period, can cause hypertension in later life. This study examined the effect of different sources of α-linolenic acid (canola oil or flaxseed oil) in the prevention of hypertension and other metabolic symptoms induced by an ω-3 fatty acid-deficient diet. Dams were provided one of three experimental diets from 1 week before mating. Diets were either deficient (10% safflower oil-DEF) or sufficient (7% safflower oil+3% flaxseed oil-SUF-F; or 10% canola oil-SUF-C) in ω-3 fatty acids. The male offspring were continued on the maternal diet from weaning for the duration of the study. Body weight, ingestive behaviors, blood pressure, body composition, metabolic rate, plasma leptin and brain fatty acids were all assessed. The DEF animals were hypertensive at 24 weeks of age compared with SUF-F or SUF-C animals; this was not evident at 12 weeks. These results suggest that different sources of ALA are effective in preventing hypertension related to ω-3 fatty acid deficiency. However, there were other marked differences between the DEF and, in particular, the SUF-C phenotype including lowered body weight, adiposity, leptin and food intake in SUF-C animals. SUF-F animals also had lower, but less marked reductions in adiposity and leptin compared with DEF animals. The differences observed between DEF, SUF-F and SUF-C phenotypes indicate that body fat and leptin may be involved in ω-3 fatty acid deficiency hypertension.
Similar content being viewed by others
Introduction
Ω-3 polyunsaturated fatty acids have diverse physiological roles in vivo including cell membrane composition1 and synthesis of eicosanoids.2 Therefore, dietary ω-3 fatty acids can act on multiple levels to alter membrane fluidity,3 inflammation4 and gene expression.5 Prenatal and life-long deficiency of the essential dietary ω-3 fatty acid, α-linolenic acid (ALA), can induce hypertension in Sprague–Dawley rats.6, 7 Deficiency can also exacerbate hypertension rats with an overactive renin-angiotensin system.8 When deficiency of dietary ω-3 fatty acid is reversed in later life brain ω-3 fatty acid levels are essentially restored,6, 7 although not in all brain membrane lipid fractions,1 but hypertension remains.6, 7 There is even some evidence that prenatal ω-3 fatty acid supplementation may reduce diastolic blood pressure in human infants.9 Overall the data indicates that prenatal ω-3 fatty acid supply has an important role in the development of the cardiovascular system and the regulation of blood pressure.
Despite this evidence, deficiency studies have not universally observed hypertension when different strains,10 sexes of rats11 or source of ω-3/ω-6 fatty acids11 have been examined. Recent evidence suggests that many of the problems with replication may be related to dietary interactions with other nutrients including protein content.12 Given the importance of other dietary factors, an examination of the effect of the dietary source of ALA on hypertension is required. The two predominant sources of ALA used in dietary studies with ω-3 fatty acids of hypertension have been canola oil (∼8% ALA)6, 7 or flaxseed oil (∼55% ALA) as part12 or all of11 the dietary fat. To further complicate the field, some studies have also examined combinations including long-chain ω-3 fatty acids.8, 10
Feeding a canola oil-rich diet can have adverse outcomes in some situations, including shortened life span,13, 14 reduced litter size and retardation of growth in stroke prone rats.15 There is also evidence that indicates canola oil may result in less body weight gain than diets high in ω-6 fatty acids.16 Thus, use of canola oil as the source of ω-3 fatty acids could act as a major confounding variable in studies of hypertension. Therefore, this study aimed to establish the effect of ALA source (canola oil or flaxseed oil) on prevention of ω-3 fatty acid deficiency-induced hypertension, and to determine if there are other effects on metabolic function in these animals.
Methods
Diets
Diets were based on American Institute of Nutrition (AIN-93G) guidelines with increased casein content (30%); the deficient diet contained 10% safflower oil (DEF); the first sufficient diet contained 7% safflower oil and 3% flaxseed oil (SUF-F); the second sufficient diet contained 10% canola oil (SUF-C). To prevent fatty acid oxidation diets contained vitamin E, were packed under nitrogen, and remained frozen before use (produced by Glen Forrest Stockfeeders, Canning Vale, WA, USA).
Animals
15 female Sprague–Dawley rats (Animal Resources Centre, Canning Vale, WA, USA) were mated with male breeders (La Trobe University, Central Animal House, Bundoora, Victoria, Australia) 1 week after being placed on one of the three experimental diets. The females remained on the diet throughout the duration of the pregnancy (21 days) and lactation (21 days). After weaning, male offspring were pair housed and continued on the same diet until the end of the experiment. There were three groups of male rats (DEF, SUF-F and SUF-C n=16/group), from five mothers/diet, chosen at random from the litters. Diets were provided ad libitum throughout the study. Food intake data was collected when animals were housed in the Labmaster indirect calorimetry system (TSE Systems, Bad Homburg, Germany). Body weights were measured by averaging balance (Sartorius, Melbourne, Victoria, Australia). All procedures were approved by the La Trobe University Animal Ethics Committee.
Tail-cuff plethysmography
Animals were acclimated to the system (Rat Blood Pressure System, IITC, Woodland Hills, CA, USA) for 2 days prior to measurements; rats were placed in restrainers in the heated (27±2 °C) system. Following the 2 acclimation days, testing was performed over 2 further days. Three recordings were taken per animal per day; the average of the six measurements was calculated for systolic blood pressure, diastolic blood pressure and heart rate. This procedure was performed at 12 and 24 weeks of age.
Dual energy X-ray absorptiometry (DEXA)
Animals were anaesthetized with Ketamine (60 mg kg–1; Troy Laboratories, Smithfield, New South Wales, Australia)/Xylazine (8 mg kg–1; Bayer, Botany, New South Wales, Australia) and laid flat on the scanning platform on their ventral side. A Norland XR-36 Dual Energy X-ray Densitometer (InMed, Baulkham Hills, New South Wales, Australia) was used to determine body fat mass, fat-free mass and fat percentage with software specifically written for small animals. This procedure was performed at 16 weeks of age.
Indirect calorimetry
Rats were placed in the calorimetry system cages for 36 h; the first 12 h was considered the acclimation phase and data were analyzed only for the final 24 h. The system used was a custom built 4-cage open-circuit calorimetry system; respiratory exchange ratio, energy expenditure, ad libitum feeding, drinking and general locomotor activity were continuously measured (LabMaster; TSE Systems). The data analyzed were respiratory exchange ratio, total energy expenditure and ingestive behaviors. These tests were performed in weeks 25–28.
Blood and tissue analysis
Leptin was analyzed in plasma using a commercially available ELISA kit (Linco Research Immunoassay, St Charles, MO, USA). Brain lipid separation was performed using thin layer chromatography and the total phospholipid fatty acids were analyzed using gas chromatography.
Statistical analysis
Data were analyzed by one-way ANOVA, followed by post hoc least significant difference test (Statistica, Statsoft) where appropriate. P<0.05 was considered to be a statistically significant difference, results are expressed as mean±s.e.m. Correlations were performed and graphs were produced using Sigmaplot (Systat Software, Chicago, IL, USA).
Results
Blood pressure
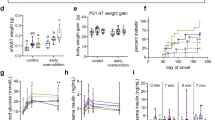
There were no differences in systolic blood pressure, diastolic blood pressure or heart rate between the groups at 12 weeks of age (data not shown). However, by 24 weeks of age the DEF group had elevated systolic blood pressure compared with both SUF groups (SUF-F P<0.01; SUF-C P<0.01); there was no difference between SUF-F and SUF-C. Diastolic blood pressure was higher at 24 weeks in DEF animals compared with SUF-F (P<0.01) and SUF-C (P<0.05) groups. There were no significant effects found for heart rate. Blood pressure results are displayed in Figure 1.
Body weight
Weaning, 16-week and 28-week body weights are displayed in Figure 2. At weaning SUF-F animals weighed significantly more than both DEF (P<0.05) and SUF-C (P<0.001) animals, the DEF group also weighed significantly more than SUF-C group (P<0.01). By 16 and 28 weeks DEF animals were significantly heavier than SUF-C (P<0.001) but not the SUF-F group; SUF-F animals also weighed more than the SUF-C group (P<0.01).
Body composition
Fat-free mass was significantly lower in SUF-C than in either DEF (P<0.05) or SUF-F (P<0.05) animals. Fat mass was highest in DEF animals followed by SUF-F animals (P<0.05) and SUF-C (P<0.001); SUF-F also had more fat than SUF-C (P<0.05). Bone mineral content was also reduced in SUF-C animals when compared with SUF-F (P<0.05) and DEF (P<0.05) animals (Figure 3). Body weight, taken manually at the time of DEXA scanning, was highly correlated with weight analyzed by DEXA (r2=0.99).
Metabolic rate
There were no differences in respiratory exchange ratio or energy expenditure during measurement in the Labmaster system (see Figure 4).
Ingestive behavior
Animals in the SUF-C group ate significantly less than both the SUF-F (P<0.01) and DEF (P<0.01) groups. Water intake followed the same pattern SUF-C animals drank less than SUF-F (P<0.01) and DEF (P<0.01) animals. Ingestive behavior results are displayed in Figure 4.
Plasma leptin
Circulating leptin levels were higher in DEF animals than in the SUF-F (P<0.05) and SUF-C (P<0.01) groups. The difference between the SUF groups was not significant (P>0.05). There was a significant correlation between plasma leptin and body fat percentage (r=0.738; P<0.001). There was also a significant correlation between plasma leptin and systolic blood pressure (r=0.549; P<0.001). Leptin results are presented in Figure 5.
Plasma leptin was increased in DEF animals (a; n=12/group). There were positive correlations between plasma leptin and body fat percentage (b; r=0.738; P<0.001; n=12/group), body fat percentage and systolic blood pressure (c; r=0.477; P<0.01; n=12/group), and plasma leptin and systolic blood pressure (d; r=0.549; P<0.001; n=12/group).
Brain fatty acids
The proportion of docosahexaenoic acid in the frontal cortex was significantly lower in the DEF group (8.41%±0.10) than both the SUF-F (9.45%±0.18 P<0.01) and SUF-C (9.39%±0.19 P<0.01) groups, with no difference between the SUF groups.
Discussion
This study shows that the hypertension induced by ω-3 fatty acid deficiency from a safflower oil-based diet can be prevented by diets containing vegetable oils rich in ALA; either a canola oil diet or a combination of flax seed oil with safflower oil. Safflower oil has been used in many different studies to induce ω-3 fatty acid deficiency due to the extremely low ALA levels in this oil (<0.5% of total fatty acids).6, 8, 12, 17 As previously reported, ω-3 deficiency-induced hypertension did not develop in DEF animals by 12 weeks of age, but was present at 24 weeks.12 The present data indicates that dietary vegetable oil source does not change the normotensive effects of ALA. However, it was not expected that the two ALA-containing diets would have significantly altered the phenotype of the animals in a number of ways including body weight and body composition.
Given that both SUF-F and SUF-C groups had less body fat, the hypertension in DEF animals may be due to an increase in adipose tissue and body fat percentage, as obesity has a well-documented effect on hypertension.18 This may be controlled by a number of factors secreted by adipose tissue. Indeed, there is some evidence to suggest that ω-3 fatty acids affect hypertension through an interaction with angiotensin (ANG) II19, 20 a hormone that can be produced in adipocytes.21 Higher levels of vasodilative eicosanoid metabolites from the ω-3 fatty acid eicosapentaenoic acid in SUF animals may be causing a reduction in the vasoconstrictive effects of ANG II as ω-3 fatty acids are known to affect vascular responses to infusion of exogenous ANG II.22, 23, 24 However, the effect of ω-3 fatty acids on hypertension cannot be entirely mediated by the eicosanoids effects on ANG II; as this would not explain the perinatal programming that is known to occur with ω-3 fatty acid deficiency6, 7, 8 and would relate only to the diet an animal is currently maintained on. Given there was no difference in fluid consumption between the SUF-F and DEF groups, and fluid intake increases with ANG II,25 this may indicate that hypertension is not due to an overactive renin-angiotensin system; however, it may be that ANG II is elevated but not to the extent that it alters drinking behavior.
The elevated leptin levels in DEF animals may be a mechanism for hypertension in DEF animals. A role for leptin as a cause of elevated blood pressure has been proposed given that elevation of blood pressure occurs in both lean and obese hyperleptinemic mice,26 and chronic peripheral, but not central,27 leptin infusion also increases mean arterial pressure in the rat.28, 29 This suggests that increases in leptin may be an underlying mechanism that contributes to hypertension in ω-3 fatty acid-deficient animals.
Given the DEF animals ate more than SUF-C animals this may indicate leptin insensitivity; however, this may relate to an increase in consumption based on body weight. Unfortunately, individual food intake could only be measured while animals were in the indirect calorimetry system as at other times they were housed two per cage due to ethical considerations. However, over the course of the study, generally, pairs of animals from the DEF and SUF-F groups ate more than pairs from the SUF-C group.
Interestingly, weaning weights of the groups did not reflect eventual body weights for SUF-F and DEF groups. This is unusual as weaning weight tends to be reflected in adult body weight,30 and low weaning weight may be associated with decreased lifespan.31 The greater increases in body weight observed resulted in greater fat mass and higher plasma leptin of the DEF group. This was most likely caused by greater food intake over time as the lack of difference in energy expenditure between groups during indirect calorimetry indicates that it was not due to changes in energy expenditure.
As expected, the concentrations of docosahexaenoic acid, the predominant ω-3 fatty acid in the brain, in the prefrontal cortex were related to the ω-3 fatty acid deficiency/sufficiency of the diet the animals were fed. Despite the difference in ALA content of the SUF-F (∼1.8%) and SUF-C (∼0.8%) diets, there was no difference in the proportion of docosahexaenoic acid in the frontal cortex of animals fed the SUF-F and SUF-C diets. This indicates that these dietary levels of ω-3 fatty acid are adequate to prevent deficiency, and hence, prevent hypertension.
Future research should extend this work by determining the role of leptin in hypertension following ω-3 fatty acid deficiency. This could be performed by infusion of a leptin antagonist and would help to determine actually causative rather than simply correlative. Also, given the proposed role for ANG II in ω-3 fatty acid deficiency hypertension12 measuring plasma renin activity, angiotensin-converting enzyme activity and ANG II would all be of interest in future work, as would assessment of ACE inhibitor treatment in this model.
Overall, the results of the current study indicate that hypertension caused by ω-3 fatty acid deficiency can be prevented by either of the two different vegetable oil sources of ALA. These data suggest a contributing cause of the hypertension induced by the ω-3 fatty acid deficiency might be related to the higher body weight, body fat and plasma leptin levels. This should be explored further in future studies.
References
Li D, Weisinger HS, Weisinger RS, Mathai M, Armitage JA, Vingrys AJ, Sinclair AJ . Omega 6 to omega 3 fatty acid imbalance early in life leads to persistent reductions in DHA levels in glycerophospholipids in rat hypothalamus even after long-term omega 3 fatty acid repletion. Prostaglandins Leukot Essent Fatty Acids 2006; 74: 391–399.
Rao JS, Ertley RN, DeMar Jr JC, Rapoport SI, Bazinet RP, Lee HJ . Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry 2007; 12: 151–157.
Litman BJ, Niu SL, Polozova A, Mitchell DC . The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways: visual transduction. J Mol Neurosci 2001; 16: 237–242; discussion 279–284.
Kremer JM, Jubiz W, Michalek A, Rynes RI, Bartholomew LE, Bigaouette J, Timchalk M, Beeler D, Lininger L . Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med 1987; 106: 497–503.
Kitajka K, Sinclair AJ, Weisinger RS, Weisinger HS, Mathai M, Jayasooriya AP, Halver JE, Puskas LG . Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc Natl Acad Sci USA 2004; 101: 10931–10936.
Armitage JA, Pearce AD, Sinclair AJ, Vingrys AJ, Weisinger RS, Weisinger HS . Increased blood pressure later in life may be associated with perinatal n-3 fatty acid deficiency. Lipids 2003; 38: 459–464.
Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS . Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nat Med 2001; 7: 258–259.
Jayasooriya AP, Begg DP, Chen N, Mathai ML, Sinclair AJ, Wilkinson-Berka J, Wark JD, Weisinger HS, Weisinger RS . Omega-3 polyunsaturated fatty acid supplementation reduces hypertension in TGR(mRen-2)27 rats. Prostaglandins Leukot Essent Fatty Acids 2008; 78: 67–72.
Forsyth JS, Willatts P, Agostoni C, Bissenden J, Casaer P, Boehm G . Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: follow up of a randomised controlled trial. BMJ 2003; 326: 953.
Martin DA, McCutcheon D, Wainwright PE . Prenatal dietary docosahexaenoic acid supplementation in combination with protein restriction does not affect blood pressure in adult Wistar rats. Nutr Neurosci 2004; 7: 141–150.
Korotkova M, Gabrielsson BG, Holmang A, Larsson BM, Hanson LA, Strandvik B . Gender-related long-term effects in adult rats by perinatal dietary ratio of n-6/n-3 fatty acids. Am J Physiol Regul Integr Comp Physiol 2005; 288: R575–R579.
Begg DP, Sinclair AJ, Stahl LA, Garg ML, Jois M, Weisinger RS . Dietary protein level interacts with omega-3 polyunsaturated fatty acid deficiency to induce hypertension. Am J Hypertens 2010; 23: 125–128.
Naito Y, Nagata T, Takano Y, Nagatsu T, Ohara N . Rapeseed oil ingestion and exacerbation of hypertension-related conditions in stroke prone spontaneously hypertensive rats. Toxicology 2003; 187: 205–216.
Ratnayake WM, L’Abbe MR, Mueller R, Hayward S, Plouffe L, Hollywood R, Trick K . Vegetable oils high in phytosterols make erythrocytes less deformable and shorten the life span of stroke-prone spontaneously hypertensive rats. J Nutr 2000; 130: 1166–1178.
Tatematsu K, Fuma SY, Satoh J, Ichikawa Y, Fujii Y, Okuyama H . Dietary canola and soybean oil fed to SHRSP rat dams differently affect the growth and survival of their male pups. J Nutr 2004; 134: 1347–1352.
Stachon M, Furstenberg E, Gromadzka-Ostrowska J . Effects of high-fat diets on body composition, hypothalamus NPY, and plasma leptin and corticosterone levels in rats. Endocrine 2006; 30: 69–74.
Weisinger H, Armitage J, Sinclair A, Vingrys A, Burns P, Weisinger R . Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nat Med 2001; 7: 258–259.
Szent-Gyorgyi N . Obesity and hypertension among young adults. Am J Clin Nutr 1957; 5: 274–278.
Juan H, Sametz W . Vasoconstriction induced by noradrenaline and angiotensin II is antagonized by eicosapentaenoic acid independent of formation of trienoic eicosanoids. Naunyn Schmiedebergs Arch Pharmacol 1986; 332: 288–292.
Juan H, Sutter D, Sametz W . Influence of eicosapentaenoic acid on noradrenaline and angiotensin-induced contractions of the rabbit aorta: mode of action. Prog Clin Biol Res 1987; 242: 57–62.
Weisinger RS, Begg DP, Chen N, Jois M, Mathai ML, Sinclair AJ . The problem of obesity: is there a role for antagonists of the renin-angiotensin system? Asia Pac J Clin Nutr 2007; 16 (Suppl 1): 359–367.
Kenny D, Warltier DC, Pleuss JA, Hoffmann RG, Goodfriend TL, Egan BM . Effect of omega-3 fatty acids on the vascular response to angiotensin in normotensive men. Am J Cardiol 1992; 70: 1347–1352.
Chin JP, Gust AP, Nestel PJ, Dart AM . Marine oils dose-dependently inhibit vasoconstriction of forearm resistance vessels in humans. Hypertension 1993; 21: 22–28.
Hui R, St-Louis J, Falardeau P . Antihypertensive properties of linoleic acid and fish oil omega-3 fatty acids independent of the prostaglandin system. Am J Hypertens 1989; 2: 610–617.
Weisinger RS, Blair-West JR, Burns P, Denton DA . Intracerebroventricular infusion of angiotensin II increases water and ethanol intake in rats. Am J Physiol 1999; 277: R162–R172.
Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K . Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest 2000; 105: 1243–1252.
Overton JM, Williams TD, Chambers JB, Rashotte ME . Central leptin infusion attenuates the cardiovascular and metabolic effects of fasting in rats. Hypertension 2001; 37: 663–669.
da Silva AA, Kuo JJ, Hall JE . Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension 2004; 43: 1312–1317.
Shek EW, Brands MW, Hall JE . Chronic leptin infusion increases arterial pressure. Hypertension 1998; 31: 409–414.
Berg BN, Simms HS, Everitt AV . Nutrition and longevity in the rat. V. Weaning weight, adult size, and onset of disease. J Nutr 1963; 80: 255–262.
Drori D, Folman Y . Interactive environmental and genetic effects on longevity in the male rat: litter size, exercise, electric shocks and castration. Exp Aging Res 1986; 12: 59–64.
Acknowledgements
This work was supported by the National Health and Medical Research Council of Australia (350313) and the Australian Research Council (LP0883996). DP Begg was supported by a Deakin University Postgraduate Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Begg, D., Sinclair, A., Stahl, L. et al. Hypertension induced by ω-3 polyunsaturated fatty acid deficiency is alleviated by α-linolenic acid regardless of dietary source. Hypertens Res 33, 808–813 (2010). https://doi.org/10.1038/hr.2010.84
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.84
Keywords
This article is cited by
-
Association of circulating saturated fatty acids with the risk of pregnancy-induced hypertension: a nested case–control study
Hypertension Research (2020)
-
Flaxseed for Hypertension: Implications for Blood Pressure Regulation
Current Hypertension Reports (2014)
-
Synergism of α‐Linolenic Acid, Conjugated Linoleic Acid and Calcium in Decreasing Adipocyte and Increasing Osteoblast Cell Growth
Lipids (2013)
-
Hypothalamic gene expression in ω-3 PUFA-deficient male rats before, and following, development of hypertension
Hypertension Research (2012)
-
Pre(peri)-natal ω-3 PUFA deficiency-induced hypertension and its broader implications
Hypertension Research (2012)