Abstract
We aimed this study to test the hypotheses that heart rate (HR) variability, evaluated by ambulatory blood pressure monitoring (ABPM), predicts risk of incident cardiovascular disease (CVD) in patients with type 2 diabetes (T2DM). ABPM was performed in 200 normotensive or hypertensive subjects with T2DM and 257 hypertensive subjects without diabetes (the mean age: 66.9±9.2 years; 38% were male). All subjects were untreated at the time of ABPM, and were followed for 67±27 months. Various measures of HR variability—standard deviation (s.d.) of HR, the root-mean-square of successive differences (RMSSD) of HR, systolic blood pressure (SBP)–HR relationships evaluated by slope and coefficients of correlation between SBP and HR—were used for the analyses. Cox proportional hazard models were used to estimate hazard ratios and 95% confidence intervals, after controlling for age, sex, body mass index, serum creatinine and 24-h SBP. During follow-up, there were 34 cardiovascular events. Awake HR variability in diabetics was smaller than non-diabetics, but sleep HR variability was similar between the groups. In multivariable analyses, increased sleep HR variability evaluated by s.d. and RMSSD of sleep HR, and slope and correlation coefficient of SBP–HR each was independently associated with the increased risk of CVD in T2DM. For non-diabetics, decreased slope of 24 h SBP–HR, and decreased correlation of 24 h SBP–HR were associated with increased risk of CVD. In conclusion, increased HR variability during sleep was a predictor for incident CVD in T2DM, but not in non-diabetics. Increased HR variability at night would reflect pathophysiological mechanism of T2DM.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2DM) is an well established risk factor for cardiovascular disease (CVD), especially when hypertension coexists.1, 2 As hypertension and T2DM synergistically act on cardiovascular risk, special attention should be paid for controlling blood pressure (BP) in patients with T2DM.3, 4 There is an increasing body of evidence that ambulatory BP is a better predictor of future CVD than clinic BP in hypertensive patients.5, 6, 7, 8, 9, 10 In T2DM, abnormal circadian rhythm of BP (non-dipper) has been reported to be associated with cardiac autonomic neuropathy (CAN),11 urinary albumin12 and cardiovascular events,13 but the clinical significance of ambulatory BP monitoring (ABPM) in T2DM is not well established.
Less variability of heart rate (HR) seen with diabetic neuropathy is applied to the standard test for the diagnosis of CAN.11, 14, 15, 16 Twenty-four hours HR variability using power spectral analysis of HR was also reported to be a sensitive test in detecting early CAN rather than standard autonomic reflex tests,17, 18, 19 and the abnormal circadian pattern of HRV was reported to be associated with excess CVD mortality rates.11 Bernardi et al.20 showed that diabetic subjects with or without signs of autonomic neuropathy have a decreased vagal activity (and hence a relatively higher sympathetic activity) during night hours. However, there have been no reports showing the prognostic significance of 24-h HR variability evaluated by ABPM in T2DM. Thus, we performed this study to test the hypothesis that increased HR variability during sleep, produced by blunted vagal tone at night in T2DM, is associated with adverse cardiovascular prognosis.
Methods
This prospective study was performed in a sample of 200 normotensive or hypertensive subjects with T2DM and 257 hypertensives without antihypertensive medications who were seen in clinics at three participating institutes in Japan: one clinic and two hospitals in the Karatsu–Nishiarita Study.21
During the period of recruitment, 1996–2002 for the Karatsu–Nishiarita Study, subjects were enrolled consecutively while being treated or evaluated for hypertension or diabetes in the clinic, and agreed to undergo ABPM. Hypertension was diagnosed when the clinic systolic BP (SBP) was ⩾140 and/or diastolic BP was ⩾90 mm Hg on at least two occasions according to current guidelines,22 or by a previous diagnosis of hypertension with current antihypertensive medication use. Clinic BP was measured at least twice on two separate occasions after at least 5 min of rest in the sitting position and after being fitted with an ABPM. Subjects took no antihypertensive medications for a minimum of 7 days before the ABPM and most took no medication during the 14 days preceding the ABPM study. T2DM was diagnosed according to the guidelines of the American Diabetes Association23 or a previous diagnosis and currently taking antidiabetic medication. We excluded patients with type 1 or secondary diabetes, renal dysfunction (serum creatinine >1.8 mg per 100 ml), hepatic damage, ischemic heart disease or other cardiac diseases, congestive heart failure, arrhythmias (including atrial fibrillation), stroke (including transient ischemic attacks) or other major concomitant non-CVDs. Those who have frequent paroxysmal ventricular, atrial ectopic beats or artifacts were not included in this study. Body mass index (BMI) was calculated as weight/height2 (kg m−2). Smoking was defined as current smoking. This study was approved by the Institutional Review Board of each participating hospital or clinic. All the subjects studied were ambulatory and gave informed consent for the study.
Ambulatory BP monitoring
Noninvasive ABPM was performed on a weekday with an automatic system (TM2421 or TM2425; A&D, Tokyo, Japan), which recorded BP by the oscillometric method and HR every 30 min for 24 h. The HR was recorded every 30 min when BP was measured. The length of the recording was about 1 min in each measurement. These devices have been previously validated.24 Awake and sleep time were defined based on patients’ diaries recorded during ABPM. Awake, sleep and 24-h BP/HR were defined as the average of all BP/HR in each category. The night–day ratio of BP and HR was calculated as sleep/awake ratio of BP and HR. Mean awake and sleep levels of SBP and diastolic BP were computed and the nocturnal BP fall (%) was calculated as (awake SBP–sleep SBP)/awake SBP. The HR variability was estimated by (1) standard deviation (s.d.), (2) SBP–HR relationship evaluated by slope, (3) correlation coefficients (R) and (4) the root-mean-square of successive differences (RMSSD), all of which were separately calculated in the awake, sleep and 24-h period. The slope was calculated by the symmetric (or bisector) regression. RMSSD was calculated as the square root of the sum of squared successive BP and HR differences. The mean square successive difference is a component of the s.d., which might be more sensitive than the s.d. itself to differences in ambulatory variability between more and less reactive individuals. The s.d. reflects all variations from the mean without regard to their sequence, whereas the mean square successive difference responds selectively to the sequential changes in HR characteristic of transient responses.25, 26 Examples of SBP–HR relationship are shown in Figure 1. As a typical case, SBP and HR in each daytime and nighttime have a linear relationship in non-diabetics (shown as slopes and correlation coefficients), but there is no such relationship in diabetics.
Follow-up and events
The subjects’ medical records were reviewed every year after ABPM for the purpose of identifying any new onset of CVD. The 457 participants enrolled in 1996–2002 for the Karatsu–Nishiarita Study were followed from March 2004 to October 2006 for up to 9.7 years. Participants who died from non-cardiovascular causes were censored as of the time of their death. The average follow-up period was 66.7±26.9 months (range: 9–120 months). When subjects did not visit the clinics, we interviewed them by telephone. We defined three outcomes: stroke, fatal or non-fatal myocardial infarction and sudden cardiac death. Stroke or cardiac events were diagnosed by the physician, caring for the patient at the time of the event and independent neurologists or cardiologists reviewed the cases and confirmed the diagnosis. Stroke was diagnosed on the basis of sudden onset of a neurological deficit that persisted for >24 h in the absence of any other disease process that could explain the symptoms. Stroke events included ischemic stroke (cerebral infarction and cerebral embolism), hemorrhagic stroke (cerebral hemorrhage and subarachnoid hemorrhage) and undefined types of stroke. We excluded transient ischemic attack, in which the neurological deficit cleared completely in <24 h.27 Myocardial infarction was diagnosed based on the AHA criterion of ‘definite’ myocardial infarction.28
Statistical analyses
All statistical analyses were carried out with SPSS/Windows, version 13.0 (SPSS, Chicago, IL, USA). The data are expressed as the mean (±s.d.) or percentage. The χ2 test was used to compare proportions. Unpaired t-test was performed to test mean differences between groups. Multivariable Cox regression analysis was performed to calculate adjusted hazard ratios with 95% confidence intervals (CI) by two steps. As a first step, age (years), sex (male=1, female=0), BMI (kg m−2), current smoking (yes or no), serum creatinine (mg per 100 ml), 24-h SBP and 24-h HR were entered in the model. On the basis of the result of step 1, we determined to use essential variables (age, sex and BMI) and possible confounding factors such as serum creatinine (hazard ratio=1.14, 95% CI=0.97–1.34, P=0.1) and 24-h SBP (hazard ratio=1.03, 95% CI=1.01–1.05, P=0.001), but not to use smoking (hazard ratio=1.30, 95% CI=0.54–3.13, P=0.6) and 24-h HR (hazard ratio=1.02, CI=0.98–1.06, P=0.4), because they were neither essential variables nor confounding factors. To examine the potential moderating effect of T2DM on the relationship of HRV to incident CVD, an interaction term (between significant measures of HRV and the presence of diabetes) was tested. The null hypothesis was rejected when two-tailed P<0.05. As the power to detect interaction effects is low, we used two-tailed P<0.10 as the criterion to judge statistical significance.
Results
The baseline characteristics are shown in Table 1. The mean age was 66.9±9.2 years; there were 172 men and 285 women; 55% of subjects were taking antihypertensive medication. The age, BMI, proportion of antihypertensive medication and ambulatory SBP were similar between diabetic and non-diabetic groups, but serum creatinine and clinic BP were lower in diabetic group. Ambulatory HR and night–day ratio of BP in diabetics were higher than those in non-diabetics.
Table 2 shows the comparison of BP variability between diabetics and non-diabetics. As shown, there were no significant differences in BP variability between diabetics and non-diabetics except for smaller s.d. of awake diastolic BP in diabetics. Sleep BP variability was similar between the groups.
We compared HR variability and SBP–HR relationships between diabetics and non-diabetics. As shown in Table 3, s.d. of awake HR, RMSSD of 24-h HR and RMSSD of awake HR were significantly lower in diabetics than in non-diabetics. However, s.d. of sleep HR variability was similar between the groups. With regard to SBP–HR relationships, there were no significant differences between the groups.
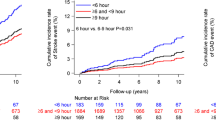
In multivariable Cox regression analyses that controlled for age, sex, BMI, serum creatinine and 24-h SBP, increased s.d. of sleep HR and RMSSD of sleep HR were significantly associated with increased incidence of CVD in diabetics (Table 4). Increased slope of SBP–HR and increased correlation coefficient of SBP–HR were also associated with the increased risk of CVD in diabetics. On the other hand, reduced correlation of 24-h SBP–HR relationship was marginally associated with increased risk of CVD in non-diabetics.
Finally, the interaction term was significant between the presence of diabetes and s.d. of sleep HR (P=0.03), RMSSD of sleep HR (P=0.02), correlation of SBP–HR during sleep (P=0.06), and slope of SBP–HR during sleep (P=0.02).
Discussion
In patients with T2DM, increased variability of HR during sleep was an independent predictor for incident CVD. On the other hand, reduced 24-h HR variability for corresponding BP change was also an independent predictor for future cardiovascular events. This study is the first study showing the predictive value of HR variability on cardiovascular prognosis in diabetics and non-diabetics from the same cohort.
Sleep HR variability in type 2 diabetics
In this study, increased HR variability during sleep was significantly associated with the increased risk of incident CVD in patients with T2DM. In addition, closer relationships between HR and SBP during sleep were associated with increased risk of CVD. Decreased HR variability has been shown to be associated with increased mortality after myocardial infarction29, 30 and cardiac events in general population.31 However, although somewhat paradoxically, increased HR variability during sleep was associated with increased risk of cardiovascular events in this study, which has rarely reported before. That was supported in this study showing that the interactions between the increased HRV measures and the presence of T2DM were significant. In diabetes, reduction of HRV during controlled conditions14, 32, 33 and 24-h ambulatory ECG15, 17, 20 is reported. It has been shown that diabetic subjects with or without signs of autonomic neuropathy have a decreased vagal activity (and hence a relatively higher sympathetic activity) during nighttime,20, 34 and which could result in reduced buffering effect of acute increase in cardiovascular overload. Therefore, diabetic patients are exposed for long period to the potentially dangerous effect of sympathetic predominance.20, 35 In a study of type 1 diabetes with autonomic neuropathy, the square root of mean squared differences of successive RR intervals showed a significant increase during nighttime compared to daytime (9.7±1.1 ms vs. 8.5±0.7 ms, P=0.02), which indicates that nocturnal predominance of parasympathetic activity is lacking in type 1 diabetes with autonomic neuropathy.36 Increased nocturnal physical activity along with sleep apnea episode would be another explanation of our result. Patients with diabetes have increased frequency of napping and is associated with nocturnal sleep fragmentation.37 Increased frequency of sleep apnea syndrome is reported in DM, and sleep apnea syndrome is reported to be associated with an increased variability of nocturnal HR.38 Nocturnal physical activity was positively associated with sleep HR,39 and s.d. of sleep activity was increased in non-dippers. Namely, altered sleep profile complicated with sleep apnea syndrome and increased nocturnal physical activity, nocturnal HR increases and could cause increased variability of HR at night. Epidemiological evidence has shown an increased number of cardiovascular events during night hours in diabetics compared with non-diabetic subjects.40, 41 Hence, enhanced HR variability caused by predominance of sympathetic tone at night might have facilitated the onset of cardiovascular accidents in our study.
Twenty-four hours HR variability in non-diabetics
In our result of non-diabetics, the less close 24-h SBP–HR relationships were associated with increased risk of incident CVD. This means that the less changes of HR for given SBP for 24 h were associated with increased cardiovascular events in non-diabetic patients. The result is in line with a previous report showing that reduced daytime HR variability was associated with cardiovascular mortality in general population.42 During 24-h ambulatory monitoring in non-diabetic subjects, HR changes along with BP changes.43 Tochikubo et al.44 have shown that there were positive correlations between 24-h SBP and 24-h HR in mild to moderate hypertension and normotensives, but the correlation was diminished in severe hypertension and secondary hypertension. In a study of ABPM in familial amyloid polyneuropathy, day–night difference of HR was maintained in patients whose parasympathetic ANS was impaired but cardiovascular sympathetic function was maintained.45 This means that discordance of 24-h BP–HR relationship will not be apparent until cardiac sympathetic nervous system is affected in addition to parasympathetic dysfunction. Therefore, those who had less close relationship between SBP and HR for 24 h could have suffered from some extent of autonomic disorder, and then ended up with poor cardiovascular outcomes.
Our main findings were that increased HR variability was a predictor of future cardiovascular events in T2DM, but not in non-diabetes. HR variability adds significantly to ambulatory BP level to predict future cardiovascular events. Even in the lower office BP, and similar ABP levels compared with non-diabetic patients, increased HR variability was associated with increased risk of cardiovascular events in diabetic patients. These findings indicate that hemodynamic change at nighttime would be very important in preventing cardiovascular events. Further studies are needed to confirm these findings and establish a novel approach.
Study limitations
There are some limitations in this study. First, HR variability was evaluated every 30 min both in the daytime and nighttime using ABPM. Compared with Holter ECG method, this method may be less accurate, however, this also enables us to evaluate 24-h BP level simultaneously and study a large number of patients. Second, we did not evaluate diabetic neuropathy with gold standard method, which could have influenced the HR variability during sleep. Moreover, unrecognized transient arrhythmia (PACs or Paf or sinus arrhythmia) at night cannot be denied because we did not perform 24-h Holter electrocardiogram. Further study is needed to compare the HRV evaluated by ABPM and gold standard test of diabetic CAN. Additionally, the clinic BP was significantly lower in the diabetic group (Table 1). The prevalence of masked hypertension (defined as normotension in the clinic but hypertension out of the clinic) was high—48% in normotensive type 2 diabetic subjects.46 Further research is needed for this issue.
Conclusions
In our diabetic population, increased HR variability during sleep was a predictor for future cardiovascular events independent of 24-h BP level and other traditional risk factors. On the other hand, in non-diabetics, decreased HR variability in 24 h was associated with the increased incidence of CVD. These results suggest that the evaluation of HR variability can be an additional risk marker of CVD, probably reflecting the early sign of diabetic and non-diabetic CAN.
References
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713.
Sowers JR . Treatment of hypertension in patients with diabetes. Arch Intern Med 2004; 164: 1850–1857.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252.
American Diabetes Association. Hypertension management in adults with diabetes. Diabetes Care 2004; 27: 65S–67S.
Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A . Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994; 24: 793–801.
Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J, for the Systolic Hypertension in Europe Trial I. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999; 282: 539–546.
Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG . Silent and clinically overt stroke in older Japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol 2001; 38: 238–245.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y . Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20: 2183–2189.
Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G . Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111: 1777–1783.
Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Hond ED, McCormack P, Staessen JA, O’Brien E . Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46: 156–161.
Spallone V, Bernardi L, Ricordi L, Solda P, Maiello MR, Calciati A, Gambardella S, Fratino P, Menzinger G . Relationship between the circadian rhythms of blood pressure and sympathovagal balance in diabetic autonomic neuropathy. Diabetes 1993; 42: 1745–1752.
Equiluz-Bruck S, Schnack C, Kopp HP, Schernthaner G . Nondipping of nocturnal blood pressure is related to urinary albumin excretion rate in patients with type 2 diabetes mellitus. Am J Hypertens 1996; 9: 1139–1143.
Nakano S, Fukuda M, Hotta F, Ito T, Ishii T, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K . Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes 1998; 47: 1501–1506.
Boulton AJM, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D . Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962.
Kudat H, Akkaya V, Sozen AB, Salman S, Demirel S, Ozcan M, Atilgan D, Yilmaz MT, Guven O . Heart rate variability in diabetes patients. J Int Med Res 2006; 34: 291–296.
Vinik AI, Ziegler D . Diabetic cardiovascular autonomic neuropathy. Circulation 2007; 115: 387–397.
Malpas SC, Maling TJ . Heart-rate variability and cardiac autonomic function in diabetes. Diabetes 1990; 39: 1177–1181.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043–1065.
Ziegler D, Piolot R . Evaluation of statistical, geometric, frequence domain, and nonlinear measures of 244-h heart rate variability in diabetic patients with various degrees of cardiovascular autonomic neuropathy. Clin Auton Res 1998; 8: 282–283.
Bernardi L, Ricordi L, Lazzari P, Soldá P, Calciati A, Ferrari MR, Vandea I, Finardi G, Fratino P . Impaired circadian modulation of sympathovagal activity in diabetes. A possible explanation for altered temporal onset of cardiovascular disease. Circulation 1992; 86: 1443–1452.
Eguchi K, Kario K, Shimada K . Greater impact of coexistence of hypertension and diabetes on silent cerebral infarcts. Stroke 2003; 34: 2471–2474.
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ . Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Circulation 2005; 111: 697–716.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004; 27: 5S–10S.
Imai Y, Sasaki S, Minami N, Munakata M, Hashimoto J, Sakuma H, Sakuma M, Watanabe N, Imai K, Sekino H, Abe K . The accuracy and performance of the A&D TM 2421, a new ambulatory blood pressure monitoring device based on the cuff-oscillometric method and the Korotkoff sound technique. Am J Hypertens 1992; 5: 719–726.
Pollak MH . Heart rate reactivity to laboratory tasks and ambulatory heart rate in daily life. Psychosom Med 1991; 53: 25–35.
Gerin W, Rosofsky M, Pieper C, Pickering TG . A test of reproducibility of blood pressure and heart rate variability using a controlled ambulatory procedure. J Hypertens 1993; 11: 1127–1131.
Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K . Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38: 852–857.
Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H . Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003; 108: 2543–2549.
Kleiger RE, Miller JP, Bigger JT, Moss AJ . Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987; 59: 256–262.
Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN . Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992; 85: 164–171.
Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D . Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996; 94: 2850–2855.
Pagani M, Malfatto G, Pierini S, Casati R, Masu AM, Poli M, Guzzetti S, Lombardi F, Cerutti S, Malliani A . Spectral analysis of heart rate variability in the assessment of autonomic diabetic neuropathy. J Auton Nerv Syst 1988; 23: 143–153.
Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D . Determinants of heart rate variability. J Am Coll Cardiol 1996; 28: 1539–1546.
Spallone V, Bernardi L, Maiello MR, Cicconetti E, Ricordi L, Fratino P, Menzinger G . Twenty-four-hour pattern of blood pressure and spectral analysis of heart rate variability in diabetic patients with various degrees of autonomic neuropathy. Comparison to standard cardiovascular tests. Clin Sci (Lond) 1996; 91 (Suppl): 105–107.
Kondo K, Matsubara T, Nakamura J, Hotta N . Characteristic patterns of circadian variation in plasma catecholamine levels, blood pressure and heart rate variability in Type 2 diabetic patients. Diabet Med 2002; 19: 359–365.
Aronson D, Weinrauch LA, D’Elia JA, Tofler GH, Burger AJ . Circadian patterns of heart rate variability, fibrinolytic activity, and hemostatic factors in type I diabetes mellitus with cardiac autonomic neuropathy. Am J Cardiol 1999; 84: 449–453.
Goldman SE, Hall M, Boudreau R, Matthews KA, Cauley JA, Ancoli-Lsrael S, Stone KL, Rubin SM, Satterfield S, Simonsick EM, Newman AB . Association between nighttime sleep and napping in older adults. Sleep 2008; 31: 733–740.
Isaksson H, Svanborg E . Obstructive sleep apnea syndrome in male hypertensives, refractory to drug therapy. Nocturnal automatic blood pressure measurements—an aid to diagnosis? Clin Exp Hypertens 1991; 13: 1195–1212.
Kario K, Schwartz JE, Pickering TG . Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension 1999; 34: 685–691.
Hjalmarson A, Gilpin EA, Nicod P, Dittrich H, Henning H, Engler R, Blacky AR, Smith SC, Ricou F, Ross J . Differing circadian patterns of symptom onset in subgroups of patients with acute myocardial infarction. Circulation 1989; 80: 267–275.
Rana JS, Mukamal KJ, Morgan JP, Muller JE, Mittleman MA . Circadian variation in the onset of myocardial infarction: effect of duration of diabetes. Diabetes 2003; 52: 1464–1468.
Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y . Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension 2000; 36: 901–906.
Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, di Rienzo M, Pedotti A, Zanchetti A . Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res 1983; 53: 96–104.
Tochikubo O, Miyazaki N, Kaneko Y . Relationship between 24-hour arterial pressure and heart rate variation in normotensives, hypertensives and patients with Shy-Drager syndrome. Jpn Circ J 1987; 51: 485–494.
Carvalho MJ, van den Meiracker AH, Boomsma F, Lima M, Freitas J, Veld AJ, Falcao de Freitas A . Diurnal blood pressure variation in progressive autonomic failure. Hypertension 2000; 35: 892–897.
Eguchi K, Ishikawa J, Hoshide S, Pickering TG, Shimada K, Kario K . Masked hypertension in diabetes mellitus: a potential risk. J Clin Hypertens (Greenwich) 2007; 9: 601–607.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eguchi, K., Schwartz, J., Pickering, T. et al. Increased heart rate variability during sleep is a predictor for future cardiovascular events in patients with type 2 diabetes. Hypertens Res 33, 737–742 (2010). https://doi.org/10.1038/hr.2010.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.61
Keywords
This article is cited by
-
Effects of sleep fragmentation and partial sleep restriction on heart rate variability during night
Scientific Reports (2023)
-
Impact of nocturnal heart rate variability on cerebral small-vessel disease progression: a longitudinal study in community-dwelling elderly Japanese
Hypertension Research (2015)
-
Role of Autonomic Reflex Arcs in Cardiovascular Responses to Air Pollution Exposure
Cardiovascular Toxicology (2015)
-
How many clinic BP readings are needed to predict cardiovascular events as accurately as ambulatory BP monitoring?
Journal of Human Hypertension (2014)
-
Blood pressure variability in relation to autonomic nervous system dysregulation: the X-CELLENT study
Hypertension Research (2012)