Abstract
We report a 10-year-old girl with Bardet–Biedl syndrome caused by a novel mutation in the Bardet–Biedl syndrome 10 (BBS10) gene. She had multiple malformations, including a dysmorphic face, postaxial polydactyly, polycystic kidney and amblyopia. She presented with typical BBS features, including intellectual disability with emotional outbursts and mild obesity. Whole-exome sequencing identified compound heterozygous mutations with NM_024685.3:c.1677C>A [p.(Tyr559*)] and c.1974T>G [p.(Tyr658*)]. To our knowledge, the latter mutation has never been reported previously.
Similar content being viewed by others
Bardet–Biedl syndrome (BBS; MIM 209900) is a rare autosomal recessive ciliopathy that is clinically characterized by obesity, pigmentary degeneration of the retina, postaxial polydactyly, genito-urinary abnormalities and intellectual disability with autistic spectrum disorder.1 To date, 21 mutations in BBS genes have been reported in patients with BBS.2,3 The cellular basis of BBS is closely linked to the dysfunction of the primary cilium.4 Here, we present a 10-year-old girl with BBS caused by a novel mutation of the BBS10 gene as confirmed by whole-exome sequencing.
The 10-year-old patient was the first child born to non-consanguineous Japanese healthy parents. The family history was unremarkable. The girl was born at 40-weeks of gestation with vacuum extraction but did not suffer any asphyxia. The birth weight was 3226 g. She exhibited dysmorphic features, including an upslanted palpebral fissure, hypertelorism, pointed chin and high arched palate as well as postaxial polydactyly of the right hand and left foot, polycystic kidneys and an atrial septum defect that was closed at 3 months of age. Her motor and intellectual developments were delayed since early childhood; she could not walk or speak a meaningful word at presentation. Brain MRI and electroencephalogram revealed no abnormalities. Various blood tests, including amino acid analysis, lactate and pyruvate, were within the normal limits. Karyotype analysis revealed a normal female karyotype of 46,XX.
Rumination was present at the age of two but disappeared spontaneously a few years later. This condition recurred at the age of nine, but faded out again at a later stage. An ophthalmologic examination revealed severe myopia with optic disk excavation but no pigmentary degeneration of the retina. She exhibited daily emotional outbreaks and sleep disturbances that required some sedative drugs, such as carbamazepine, cyproheptadine and risperidone, since early childhood. Mild obesity was evident, although neither type 2 diabetes nor hypertension was recognized. An echogram of the kidneys at the age of 10 years identified a few cysts around the renal pelvis and a high echoic area in the medulla of the bilateral well-developed kidneys. The estimated GFR was normal for her age, which suggests stable kidney function.
During the course of a whole-exome analysis, informed consent from the parents and approval from the University of Tsukuba Hospital review board were obtained for the molecular studies. DNA was extracted from peripheral blood samples obtained from the patient and her parents. A whole-exome analysis was performed using a HiSeq 2500 platform (Illumina, San Diego, CA, USA) and a SureSelectXT Human All Exon Kit V6 (Agilent Technologies, Santa Clara, CA, USA). The sequencing reads were aligned to the reference human genome sequence (hs37d5) using Burrows-Wheeler Transform 3 and local realignment around the indels, and base quality score recalibration was performed using the Genome Analysis Toolkit 4. Duplicate reads were removed using Picard (http://picard.sourceforge.net). Nonsynonymous coding variants, splice acceptor and donor site variants, and frameshift-coding indels were filtered against dbSNP137, the 1000 Genomes Project (http://www.1000genomes.org/), ESP6500, Japanese Genome Variation Database (https://ijgvd.megabank.tohoku.ac.jp/) or the Japanese SNP dataset of 1208 normal individuals (Human Genetic Variation Browser: http://www.genome.med-kyoto-u.ac.jp/SnpDB). The mean coverage was more than 60 reads.
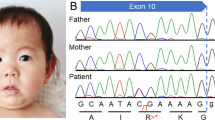
Whole-exome sequencing revealed compound heterozygous mutations with NM_024685.3:c.1677C>A [p.(Tyr559*)] and c.1974T>G [p.(Tyr658*)] in BBS10 (OMIM #615987), which were inherited from each of the parents (Figure 1). To our knowledge, the latter mutation has never been reported previously.
Sanger sequences of the patient and parents in BBS10 and predicted amino acid sequences. (a) The patient and mother have NM_024685.3:c.1677C>A mutation (arrow), which causes the termination of the BBS10 protein. (b) The patient and father have NM_024685.3:c.1974T>G mutation (arrow), which causes the termination of the protein. Thus, the patient has compound heterozygous mutations in the BBS10 gene.
Mutations in at least 21 BBS genes have been identified thus far in BBS.2,3 These BBS genes play a crucial role in the formation and function of the cilium, which is required for Hedgehog and Wnt signal transduction.4,5 Eight BBS proteins (BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, BBS9 and BBIP10/BBS18) physically interact to form a complex ‘BBSome,’ which functions as a coat for vesicles destined to the cilium, whereas BBS10 forms a CCT/TRiC/BBS chaperonin complex with BBS6 and BBS12 to mediate the assembly of the BBSome.4,6
The BBS10 protein has three chaperonin functional domains: the equatorial domain, the intermediate domain and the apical domain.7 One mutation (NM_024685.3:c.1677C>A) causes the termination of the BBS10 protein in the intermediate domain,8 and another (c.1974T>G) causes termination in the equatorial domain, which is responsible for ATP binding and hydrolysis, to act on non-native polypeptides and facilitate their folding or unfolding.7
Approximately 16 percent of BBS cases result from mutations in the BBS10 gene.9 Although there are no apparent phenotypic differences between patients with mutations in genes associated with the BBSome and those with mutations associated with the chaperonin complex,1 previous reports of patients with mutations in the BBS10 gene and of BBS10 null mutant mice suggest that there could be some tendency for differences in clinical symptoms. The BBS10−/− mouse exhibits obesity, retinal degeneration, structural defects in the glomeruli, polyuria associated with high circulating arginine vasopressin concentrations, and vacuolated, yet ciliated, renal epithelial cells.10 Additionally, severe renal disease has been reported in French patients with mutations in the BBS6, BBS10 and BBS12 genes.11
We compared the clinical symptoms in our patient with those of patients who were reported on in a review article (Table 1).2 Our patient lacked pigmentary degeneration of the retina and hypogonadism, which are the primary features of BBS. Although our patient developed severe myopia with optic disk excavation since early childhood, a detailed fundus examination did not show retinal degeneration so far at the age of 10 years. An electroretinogram has yet to be performed. Because 93% of patients with BBS exhibit retinal degeneration, a serial ophthalmologic examination is needed.1 Because the patient is at the pre-pubertal age, tests for hypogonadism were not performed. With regard to the secondary features, the facial dysmorphism observed in our patient was compatible with that reported previously.1 Behavioral problems might not be a main feature of patients with BBS10 gene mutations.2 Our patient has displayed violent emotional outbreaks and sleep disturbances since the age of 2 years; various medications were used to treat these symptoms. Furthermore, rumination, which has not been reported previously in BBS, was observed during early and later childhood, but the patient did not vomit during rumination, and the latter did not affect body growth. Further studies are needed to determine the relationship between BBS10 gene mutations and rumination.
In conclusion, we identified a novel BBS10 mutation (NM_024685.3:c.1974T>G, [p.(Tyr658*)]) in a Japanese girl who presented with relatively typical symptoms of BBS.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
References
Forsythe E, Beales PL . Bardet-Biedl syndrome. Eur J Hum Genet 2013; 21: 8–13.
Khan SA, Muhammad N, Khan MA, Kamal A, Rehman ZU, Khan S . Genetics of Human Bardet-Biedl Syndrome, an Updates. Clin Genet 2016; 90: 3–15.
Heon E, Kim G, Qin S, Garrison JE, Tavares E, Vincent A et al. Mutations in C8ORF37 cause Bardet Biedl syndrome (BBS21). Hum Mol Genet 2016; 25: 2283–2294.
Novas R, Cardenas-Rodriguez M, Irigoin F, Badano JL . Bardet-Biedl syndrome: Is it only cilia dysfunction? FEBS Lett 2015; 589: 3479–3491.
Cardenas-Rodriguez M, Badano JL . Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet 2009; 151C: 263–280.
Saibil H . Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 2013; 14: 630–642.
Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet 2006; 38: 521–524.
Billingsley G, Bin J, Fieggen KJ, Duncan JL, Gerth C, Ogata K et al. Mutations in chaperonin-like BBS genes are a major contributor to disease development in a multiethnic Bardet-Biedl syndrome patient population. J Med Genet 2010; 47: 453–463.
Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ et al. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell 2011; 22: 3289–3305.
Cognard N, Scerbo MJ, Obringer C, Yu X, Costa F, Haser E et al. Comparing the Bbs10 complete knockout phenotype with a specific renal epithelial knockout one highlights the link between renal defects and systemic inactivation in mice. Cilia 2015; 4: 10.
Imhoff O, Marion V, Stoetzel C, Durand M, Holder M, Sigaudy S et al. Bardet-Biedl syndrome: a study of the renal and cardiovascular phenotypes in a French cohort. Clin J Am Soc Nephrol 2011; 6: 22–29.
Data Citations
Ohto, Tatsuyuki HGV Database http://dx.doi.org/10.6084/m9.figshare.hgv.1387 (2017)
Ohto, Tatsuyuki HGV Database http://dx.doi.org/10.6084/m9.figshare.hgv.1390 (2017)
Acknowledgements
This research is funded by the Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Ohto, T., Enokizono, T., Tanaka, R. et al. A novel BBS10 mutation identified in a patient with Bardet–Biedl syndrome with a violent emotional outbreak. Hum Genome Var 4, 17033 (2017). https://doi.org/10.1038/hgv.2017.33
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/hgv.2017.33
This article is cited by
-
Bardet-Biedl Syndrome: a case report of delayed diagnosis with variable presentation and role of genetic testing in definitive diagnosis
Egyptian Pediatric Association Gazette (2023)
-
Bardet–Biedl syndrome and related disorders in Japan
Journal of Human Genetics (2020)
-
Characteristics of genotype and phenotype in Chinese patients with Bardet–Biedl syndrome
International Ophthalmology (2020)