Abstract
The L1264P and R1275L heterozygous mutations of the myosin heavy chain 11 (MYH11) gene, which are on the same allele, have been reported to cause thoracic aortic aneurysms and/or dissections (TAAD) complicated with patent ductus arteriosus (PDA); however, their contributions to the pathogenesis of TAAD/PDA have not been elucidated. Here we report the first familial case of TAAD with only a MYH11 L1264P mutation, in which PDA was not observed, indicating that L1264P, not R1275L, is responsible for TAAD formation.
Similar content being viewed by others
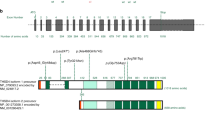
Non-syndromic thoracic aortic aneurysms and/or dissections (TAADs) are typically inherited in an autosomal dominant manner in up to 20% of cases1 and are caused by mutations of the ACTA2 and MYH11 genes, which encode smooth muscle cell (SMC)-specific isoforms of the α-actin (ACTA2) and myosin heavy chain (MYH11) genes, respectively. Mutations in the ACTA2 gene account for the majority of familial TAADs (14%),2 whereas mutations in the MYH11 gene have been reported in eight European or American familial cases3–7 and two Japanese familial cases;8 they are typically found in patients with patent ductus arteriosus (PDA). Pannu H et al.4 reported a TAAD/PDA American familial case with two missense variants, L1264P (3791T>C) and R1275L (3824G>T) of the MYH11 (NM_002474.2; NP_002465.1) gene, which are on the same allele judged by co-segregation across generational lines. The variants co-segregated with the TAAD phenotype with complete penetrance and with the PDA phenotype with reduced penetrance (50%). They have been conserved in the evolution of vertebrates from amphibians to humans (Figure 1a) in the α-helical coiled-coil domain. The COILS prediction program9 (http://www.ch.embnet.org/software/COILS_form.html), which compares the query sequence with a database of known parallel two-stranded coiled coils, suggests that L1264P affects the probability of coiled-coil formation whereas R1275L does not. The SIFT (http://sift.jcvi.org), MutationTaster (http://www.mutationtaster.org) and PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2) programs, which predict potential pathogenicity, indicated that both mutations of the MYH11 gene are probably damaging (Figure 1a). Both variants are absent from public variant databases, such as the dbSNP (http://www.ncbi.nlm.nih.gov/SNP/), Exome Variant Server (http://evs.gs.washington.edu/EVS/), Exome Aggregation Consortium (http://exac.broadinstitute.org), and Human Genetic Variation Browser (http://www.genome.med.kyoto-u.ac.jp/SnpDB/) databases. Thus, the precise contribution of each mutation to a phenotype of TAAD/PDA remains elusive. Here, we present the first familial case of non-syndromic TAADs without PDA, in which only the L1264P mutation of the MYH11 gene was identified.
Japanese familial case of TAAD with an L1264P mutation of MYH11 gene. (a) (Upper panel) Alignment of a partial sequence of human MYH11 protein (NP_002465.1) with nine protein sequences from species of vertebrates. (Lower panel) Scores from the prediction algorithms (SIFT, MutationTaster and Polyphen-2 (version 2.2.2)) indicating that L1264P (3791T>C) and R1275L (3824G>T) mutations of MYH11 (NM_002474.2; NP_002465.1), identified in an American family with TAAD/PDA,4 are probably damaging. The mutated residues are labeled on the protein sequences as indicated in the upper panel. (b) A Japanese familial case of TAAD with an L1264P mutation of the MYH11 gene. The age is shown in the upper left corner, and ‘d’ indicates the age at death. The plus and minus symbols in the upper right corner indicate individuals with and without the mutation, respectively. Square, male; circle, female; arrow, proband; question mark, insufficient clinical information; slash line, died. (c) Genomic DNA sequencing revealed a heterozygous T to C change at nucleotide 3791 of MYH11 (NM_002474.2) in the proband (III-1), whereas the nucleotide G at position 3824 was not changed.
This genetic analysis of TAADs was approved by the University of Tokyo Hospital ethics committee (G-1538). Genomic DNA was extracted from peripheral blood or saliva. Mutation analyses for the thoracic aortic aneurysm-related genes (FBN1, TGFBR1, TGFBR2, TGFB2, SMAD3, ACTA2 and MYH11) were performed, and the PCR products were sequenced with the BigDye Terminator v3.1 cycle-sequencing system on an ABI Prism 3100×l Genetic Analyzer (Applied Biosystems, Waltham, MA, USA). The sequencing was performed using forward and reverse primers. All the primer sequences are available on request. The causal mutation was defined to be co-segregating with the phenotype in the family members and unreported as a single nucleotide polymorphism (SNP) in public genomic databases.
The proband (III-1) was a 40-year-old man without any history of treatment. At the age of 39, the subject developed a sudden onset of sharp precordial pain while watching his son’s soccer game. He was diagnosed with an acute type A aortic dissection, and a Bentall operation was performed in a university hospital. Because his paternal grandmother (I-2), father (II-1), paternal uncle (II-3) and younger brother (III-3) had suffered from TAADs (Figure 1b), he visited our hospital for genetic counseling. His height and weight were 172 cm and 61 kg, respectively (body mass index 20.6), and his arm span was 183 cm (span/height ratio=1.06). There were no other skeletal, ocular or skin features typical of Marfan-related disorders, and transthoracic echocardiography revealed no cardiac abnormalities. In addition, his father (II-1) and younger brother (III-3) did not have PDA or other congenital heart diseases.
We performed genetic analyses of mutations of the TAAD-related genes including FBN1, TGFBR1, TGFBR2, SMAD3, TGFB2, ACTA2 and MYH11, among his family members and concluded that the L1264P (3791T>C) variant within exon 28 of the MYH11 gene was likely responsible for causing the TAAD phenotype (Figure 1c). The mutation was reported in an American TAAD/PDA family with L1264P and R1275L4 and is absent from public SNP databases; co-segregation of this genetic variant with the TAAD phenotype was clearly shown in all the adult patients examined here. Although one 10-year-old child of the proband with the same mutation did not have aortic dilatation on transthoracic echocardiography (sinuses of Valsalva 21.3 mm, Z-score 0.43) or magnetic resonance angiography (maximum ascending aortic diameter, 20.6 mm), he required careful follow-up because the natural history of familial TAAD has not yet been elucidated.
TAADs are linked to high mortality rates and elevated medical expenses, and it is important to identify high-risk patients and take preventative action. The MYH11 gene encodes the SMC-specific myosin heavy chain (MYH11), a major component of the contractile unit in SMCs, and the mutations have been reported to be associated with TAAD and PDA. In an American family, the same MYH11 allelic variants, L1264P and R1275L, which were located at a mutational hot-spot in exon 28 encoding the α-helical coiled-coil domain to facilitate filament assembly, co-segregated with the TAAD phenotype with complete penetrance.4 Although L1264P was predicted to be more harmfully influential on the structure or function of myosin thick filaments by COILS, an in silico prediction program for coiled-coil formation and more deleterious by the MutationTaster and PolyPhen-2 programs for mutation prediction (Figure 1a), these effects have not been conclusively shown. This case of TAAD with only L1264P provides conclusive evidence of the causality of TAAD formation, although a possibility remains that R1275L functioned as a genetic modifier.
PDA is caused by impaired oxygen-induced vasoconstriction and is highly associated with TAAD caused by MYH11 mutations, suggesting the impairment of the contractile machinery in SMCs in a TAAD/PDA family. To the best of our knowledge, PDA has been linked to at least 8 families in 10 TAAD familial cases with MYH11 mutations. In this familial case, PDA was not found in all the relatives we examined, and two of four patients in the United States with L1264P and R1275L showed PDA.4 The co-existence of PDA might be caused by L1264P alone, as a variant with inherent variable expressivity, or in combination with co-existing modifiers including R1275L. In addition, whether R1275L is totally unrelated or functions as a genetic modifier in the pathogenesis of PDA remains to be elucidated, and further research is necessary to investigate the relationship between MYH11 mutations and TAAD/PDA.
We reported the first familial case of TAAD with a MYH11 L1264P mutation in which PDA was not observed. Further genetic analyses are warranted to establish the clinical usefulness of re-sequencing the MYH11 gene to clarify a genetic background in TAAD cases.
References
References
Pyeritz RE . Heritable thoracic aortic disorders. Curr Opin Cardiol 2014; 29: 97–102.
Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39: 1488–1493.
Kathiravel U, Keyser B, Hoffjan S, Kotting J, Muller M, Sivalingam S et al. High-density oligonucleotide-based resequencing assay for mutations causing syndromic and non-syndromic forms of thoracic aortic aneurysms and dissections. Mol Cell Probes 2013; 27: 103–108.
Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet 2007; 16: 2453–2462.
Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 2006; 38: 343–349.
Renard M, Callewaert B, Baetens M, Campens L, MacDermot K, Fryns JP et al. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFbeta signaling in FTAAD. Int J Cardiol 2013; 165: 314–321.
Harakalova M, van der Smagt J, de Kovel CG, Van't Slot R, Poot M, Nijman IJ et al. Incomplete segregation of MYH11 variants with thoracic aortic aneurysms and dissections and patent ductus arteriosus. Eur J Hum Genet 2013; 21: 487–493.
Imai Y, Morita H, Takeda N, Miya F, Hyodo H, Fujita D et al. A deletion mutation in myosin heavy chain 11 causing familial thoracic aortic dissection in two Japanese pedigrees. Int J Cardiol 2015; 195: 290–202.
Lupas A, Van Dyke M, Stock J . Predicting coiled coils from protein sequences. Science 1991; 252: 1162–1164.
Data Citations
Takeda, Norifumi HGV Database (2015) http://dx.doi.org/10.6084/m9.figshare.hgv.668
Acknowledgements
We thank the patient and his or her family for their participation in this study. We also thank Ms Ikuyo Yamamoto for her excellent technical assistance. This study was supported by a Grant-in-Aid for Research on Rare and Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (NT) and a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (HM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Takeda, N., Morita, H., Fujita, D. et al. A deleterious MYH11 mutation causing familial thoracic aortic dissection. Hum Genome Var 2, 15028 (2015). https://doi.org/10.1038/hgv.2015.28
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/hgv.2015.28
This article is cited by
-
Detection of the Copy Number Variants of Genes in Patients with Familial Cardiac Diseases by Massively Parallel Sequencing
Molecular Diagnosis & Therapy (2023)
-
Basement membrane collagen IV deficiency promotes abdominal aortic aneurysm formation
Scientific Reports (2021)
-
Activation of TGF-β signaling in an aortic aneurysm in a patient with Loeys-Dietz syndrome caused by a novel loss-of-function variant of TGFBR1
Human Genome Variation (2019)
-
Compound heterozygous variants in MYH11 underlie autosomal recessive megacystis-microcolon-intestinal hypoperistalsis syndrome in a Chinese family
Journal of Human Genetics (2019)
-
Leveraging linkage evidence to identify low-frequency and rare variants on 16p13 associated with blood pressure using TOPMed whole genome sequencing data
Human Genetics (2019)