Abstract
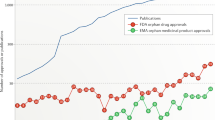
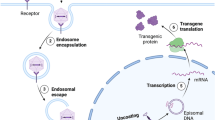
For the last 20 years, academic research has been the major, and often only, driving force behind the spectacular development of gene transfer technology for the therapy of rare genetic diseases. Investors and industry became eventually interested in gene and cell therapy, due to the success of a series of pioneering clinical trials that proved efficacy and safety of last-generation technology, and to favorable orphan drug legislation in both Europe and the United States. Developing this forms of therapy is however complex and requires skills and knowledge not necessary available to the industry, which is better placed to develop processes and products and put them on the market. Cooperation between academia and industry is an opportunity to de-risk innovative approaches and ensure a faster and more economical development of therapies for diseases with high unmet medical needs and low-profit expectations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Abbot A . Gene therapy. Italians first to use stem cells. Nature 1992; 356: 465.
Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science 1995; 270: 470–475.
Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science 1995; 270: 475–480.
GSK Press Release Available at: http://www.gsk.com/en-gb/media/press-releases/2016/strimvelistm-receives-european-marketing-authorisation-to-treat-very-rare-disease-ada-scid/> 2016.
Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002; 296: 2410–2413.
Aiuti A, Vai S, Mortellaro A, Casorati G, Ficara F, Andolfi G et al. Immune reconstitution in ADA-SCID after PBL gene therapy and discontinuation of enzyme replacement. Nat Med 2002; 8: 423–425.
Aiuti A, Cassani B, Andolfi G, Mirolo M, Biasco L, Recchia A et al. Multilineage hematopoietic reconstitution without clonal selection in ADA-SCID patients treated with stem cell gene therapy. J Clin Invest 2007; 117: 2233–2240.
Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 2009; 360: 447–458.
Cavazza A, Moiani A, Mavilio F . Mechanisms of retroviral integration and mutagenesis. Hum Gene Ther 2013; 24: 119–131.
Basner-Tschakarjan E, Mingozzi F . Cell-mediated immunity to AAV Vectors, evolving concepts and potential solutions. Front Immunol 2014; 5: 350.
Naldini L . Gene therapy returns to centre stage. Nature 2015; 526: 351–360.
Meekings KN, Williams CS, Arrowsmith JE . Orphan drug development: an economically viable strategy for biopharma R&D. Drug Discov Today 2012; 17: 660–664.
Lim WA, June CH . The principles of engineering immune cells to treat cancer. Cell 2017; 168: 724–740.
Bryant LM, Christopher DM, Giles AR, Hinderer C, Rodriguez JL, Smith JB et al. Lessons learned from the clinical development and market authorization of Glybera. Hum Gene Ther Clin Dev 2013; 24: 55–64.
Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G . Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010; 363: 147–155.
GSK Press Release. Available at: https://us.gsk.com/en-us/media/press-releases/2010/gsk-fondazione-telethon-and-fondazione-san-raffaele-to-collaborate-on-gene-therapy-for-rare-diseases/> 2010.
Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 2013; 341: 1233151.
Hacein-Bey Abina S, Gaspar HB, Blondeau J, Caccavelli L, Charrier S, Buckland K et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA 2015; 313: 1550–1563.
Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013; 341: 1233158.
Audentes Press Release. Available at: http://investors.audentestx.com/phoenix.zhtml?c=254280&p=irol-newsArticle&ID=2123663> 2014.
Childers MK, Joubert R, Poulard K, Moal C, Grange RW, Doering JA et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med 2014; 6: 220ra10.
Check Heyden E . Gene therapies pose million-dollar conundrum. Nature 2016; 534: 305–306.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Mavilio, F. Developing gene and cell therapies for rare diseases: an opportunity for synergy between academia and industry. Gene Ther 24, 590–592 (2017). https://doi.org/10.1038/gt.2017.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.36
This article is cited by
-
Engineering the next generation of cell-based therapeutics
Nature Reviews Drug Discovery (2022)
-
Reconsidering clinical pharmacology frameworks as a necessary strategy for improving the health care of patients: a systematic review
European Journal of Clinical Pharmacology (2018)
-
Rare Diseases: Drug Discovery and Informatics Resource
Interdisciplinary Sciences: Computational Life Sciences (2018)