Abstract
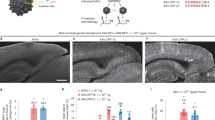
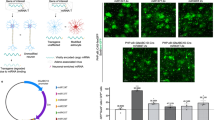
No adeno-associated virus (AAV) capsid has been described in the literature to exhibit a primary oligodendrocyte tropism when a constitutive promoter drives gene expression, which is a significant barrier for efficient in vivo oligodendrocyte gene transfer. The vast majority of AAV vectors, such as AAV1, 2, 5, 6, 8 or 9, exhibit a dominant neuronal tropism in the central nervous system. However, a novel AAV capsid (Olig001) generated using capsid shuffling and directed evolution was recovered after rat intravenous delivery and subsequent capsid clone rescue, which exhibited a >95% tropism for striatal oligodendrocytes after rat intracranial infusion where a constitutive promoter drove gene expression. Olig001 contains a chimeric mixture of AAV1, 2, 6, 8 and 9, but unlike these parental serotypes after intravenous administration Olig001 has very low affinity for peripheral organs, especially the liver. Furthermore, in mixed glial cell cultures, Olig001 exhibits a 9-fold greater binding when compared with AAV8. This novel oligodendrocyte-preferring AAV vector exhibits characteristics that are a marked departure from previously described AAV serotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS . Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol Ther 2010; 18: 1458–1461.
Kantor B, McCown T, Leone P, Gray SJ . Clinical applications involving CNS gene transfer. Adv Genet 2014; 87: 71–124.
Kantor B, Bailey RM, Wimberly K, Kalburgi SN, Gray SJ . Methods for gene transfer to the central nervous system. Adv Genet 2014; 87: 125–197.
McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med 2006; 8: 577–588.
Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther 2010; 18: 1731–1735.
Rafii MS, Baumann TL, Bakay RA, Ostrove JM, Siffert J, Fleisher AS et al. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimers Dement 2014; 10: 571–581.
Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 2004; 10: 302–317.
Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA 2000; 97: 3428–3432.
Harding TC, Dickinson PJ, Roberts BN, Yendluri S, Gonzalez-Edick M, Lecouteur RA et al. Enhanced gene transfer efficiency in the murine striatum and an orthotopic glioblastoma tumor model, using AAV-7- and AAV-8-pseudotyped vectors. Hum Gene Ther 2006; 17: 807–820.
Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM . Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther 2006; 13: 517–527.
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK . Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 2009; 27: 59–65.
Cearley CN, Wolfe JH . Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther 2006; 13: 528–537.
Cearley CN, Vandenberghe LH, Parente MK, Carnish ER, Wilson JM, Wolfe JH . Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol Ther 2008; 16: 1710–1718.
San Sebastian W, Samaranch L, Heller G, Kells AP, Bringas J, Pivirotto P et al. Adeno-associated virus type 6 is retrogradely transported in the non-human primate brain. Gene Therapy 2013; 20: 1178–1183.
Chen H, McCarty DM, Bruce AT, Suzuki K, Suzuki K . Gene transfer and expression in oligodendrocytes under the control of myelin basic protein transcriptional control region mediated by adeno-associated virus. Gene Therapy 1998; 5: 50–58.
Lawlor PA, Bland RJ, Mouravlev A, Young D, During MJ . Efficient gene delivery and selective transduction of glial cells in the mammalian brain by AAV serotypes isolated from nonhuman primates. Mol Ther 2009; 17: 1692–1702.
Gray SJ, Blake BL, Criswell HE, Nicolson SC, Samulski RJ, McCown TJ et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB). Mol Ther 2010; 18: 570–578.
Agbandje-McKenna M, Kleinschmidt J . AAV capsid structure and cell interactions. Methods Mol Biol 2011; 807: 47–92.
Govindasamy L, Padron E, McKenna R, Muzyczka N, Kaludov N, Chiorini JA et al. Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J Virol 2006; 80: 11556–11570.
Nonnenmacher M, Weber T . Intracellular transport of recombinant adeno-associated virus vectors. Gene Therapy 2012; 19: 649–658.
Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat Biotechnol 2010; 28: 79–82.
Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther 2012; 20: 443–455.
Pulicherla N, Shen S, Yadav S, Debbink K, Govindasamy L, Agbandje-McKenna M et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther 2011; 19: 1070–1078.
Hughes EG, Kang SH, Fukaya M, Bergles DE . Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci 2013; 16: 668–676.
Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 2013; 16: 571–579.
Gray SJ, Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J et al. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Gene Ther 2011; 22: 1143–1153.
Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol Ther 2007; 15: 481–491.
Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther 2007; 18: 195–206.
Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP . AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther 2008; 16: 89–96.
Paneda A, Vanrell L, Mauleon I, Crettaz JS, Berraondo P, Timmermans EJ et al. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum Gene Ther 2009; 20: 908–917.
Xiao PJ, Li C, Neumann A, Samulski RJ . Quantitative 3D tracing of gene-delivery viral vectors in human cells and animal tissues. Mol Ther 2012; 20: 317–328.
Li W, Asokan A, Wu Z, Van Dyke T, DiPrimio N, Johnson JS et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol Ther 2008; 16: 1252–1260.
Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R . Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Therapy 2013; 20: 450–459.
Paxinos G, Watson C The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA, USA, 2014.
McCarthy KD, de Vellis J . Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 1980; 85: 890–902.
Acknowledgements
These studies were supported by Michael J. Fox Foundation and Target ALS grants to TJM, as well as a grant from the Legacy of Angels to SJG. Indirect administrative support for SJG was provided by Research to Prevent Blindness to the UNC Department of Ophthalmology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
SJG and TJM are inventors for a filed patent by UNC that includes the Olig001 capsid and is licensed to Asklepios Biopharmaceutical. They have received royalties from Asklepios Biopharmaceutical for this patent. RJS is a scientific co-founder of Asklepios Biopharmaceutical. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Powell, S., Khan, N., Parker, C. et al. Characterization of a novel adeno-associated viral vector with preferential oligodendrocyte tropism. Gene Ther 23, 807–814 (2016). https://doi.org/10.1038/gt.2016.62
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.62
This article is cited by
-
IFNγ drives neuroinflammation, demyelination, and neurodegeneration in a mouse model of multiple system atrophy
Acta Neuropathologica Communications (2024)
-
Strategies for delivering therapeutics across the blood–brain barrier
Nature Reviews Drug Discovery (2021)
-
A historical review of multiple system atrophy with a critical appraisal of cellular and animal models
Journal of Neural Transmission (2021)
-
T cell infiltration in both human multiple system atrophy and a novel mouse model of the disease
Acta Neuropathologica (2020)
-
Two-photon probes for in vivo multicolor microscopy of the structure and signals of brain cells
Brain Structure and Function (2018)