Abstract
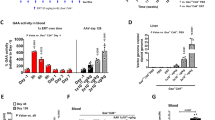
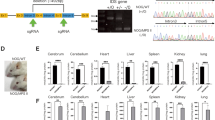
Pompe disease (glycogen storage disease type II (GSD-II)) is a myopathy caused by a genetic deficiency of acid α-glucosidase (GAA) leading to lysosomal glycogen accumulation causing muscle weakness, respiratory insufficiency and death. We previously demonstrated in GSD-II mice that a single injection of a helper-dependent adenovirus (HD-Ad) expressing GAA resulted in at least 300 days of liver secretion of GAA, correction of the glycogen storage in cardiac and skeletal muscles and improved muscle strength. Recent reports suggest that gene therapy modeling for lysososomal storage diseases in mice fails to predict outcomes in larger animal models. We therefore evaluated an HD-Ad expressing GAA in non-human primates. The baboons not only tolerated the procedure well, but the results also confirmed that a single dose of the HD-Ad allowed the livers of the treated animals to express and secrete large amounts of GAA for at least 6 months, at levels similar to those achieved in mice. Moreover, we detected liver-derived GAA in the heart, diaphragm and skeletal muscles of the treated animals for the duration of the study at levels that corrected glycogen accumulation in mice. This work validates our proof-of-concept studies in mice, and justifies future efforts using Ad-based vectors in Pompe disease patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Futerman AH, van Meer G . The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol 2004; 5: 554–565.
Rastall DP, Amalfitano A . Recent advances in gene therapy for lysosomal storage disorders. Appl Clin Genet 2015; 8: 157–169.
Meikle PJ, Hopwood JJ, Clague AE, Carey WF . Prevalence of lysosomal storage disorders. JAMA 1999; 281: 249–254.
Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med 2001; 3: 132–138.
Fratantoni JC, Hall CW, Neufeld EF . Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science 1968; 162: 570–572.
Kiang A, Hartman ZC, Liao S, Xu F, Serra D, Palmer DJ et al. Fully deleted adenovirus persistently expressing GAA accomplishes long-term skeletal muscle glycogen correction in tolerant and nontolerant GSD-II mice. Mol Ther 2006; 13: 127–134.
Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 2005; 12: 876–884.
Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 2005; 11: 57–65.
Xu F, Ding E, Migone F, Serra D, Schneider A, Chen Y-T et al. Glycogen storage in multiple muscles of old GSD-II mice can be rapidly cleared after a single intravenous injection with a modified adenoviral vector expressing hGAA. J Gene Med 2005; 7: 171–178.
Xu F, Ding E, Liao SX, Migone F, Dai J, Schneider A et al. Improved efficacy of gene therapy approaches for Pompe disease using a new, immune-deficient GSD-II mouse model. Gene Therapy 2004; 11: 1590–1598.
McVie-Wylie AJ, Ding EY, Lawson T, Serra D, Migone FK, Pressley D et al. Multiple muscles in the AMD quail can be ‘cross-corrected’ of pathologic glycogen accumulation after intravenous injection of an [E1-, polymerase-] adenovirus vector encoding human acid-alpha-glucosidase. J Gene Med 2003; 5: 399–406.
Sun B, Chen Y-T, Bird A, Xu F, Hou Y-X, Amalfitano A et al. Packaging of an AAV vector encoding human acid alpha-glucosidase for gene therapy in glycogen storage disease type II with a modified hybrid adenovirus-AAV vector. Mol Ther 2003; 7: 467–477.
Sun B, Chen Y-T, Bird A, Amalfitano A, Koeberl DD . Long-term correction of glycogen storage disease type II with a hybrid Ad-AAV vector. Mol Ther 2003; 7: 193–201.
Ding EY, Hodges BL, Hu H, McVie-Wylie AJ, Serra D, Migone FK et al. Long-term efficacy after [E1-, polymerase-] adenovirus-mediated transfer of human acid-alpha-glucosidase gene into glycogen storage disease type II knockout mice. Hum Gene Ther 2001; 12: 955–965.
Amalfitano A, McVie-Wylie AJ, Hu H, Dawson TL, Raben N, Plotz P et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci USA 1999; 96: 8861–8866.
Ding E, Hu H, Hodges BL, Migone F, Serra D, Xu F et al. Efficacy of gene therapy for a prototypical lysosomal storage disease (GSD-II) is critically dependent on vector dose, transgene promoter, and the tissues targeted for vector transduction. Mol Ther 2002; 5: 436–446.
Ziegler RJ, Lonning SM, Armentano D, Li C, Souza DW, Cherry M et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther 2004; 9: 231–240.
Koeberl DD, Sun B, Bird A, Chen YT, Oka K, Chan L . Efficacy of helper-dependent adenovirus vector-mediated gene therapy in murine glycogen storage disease type Ia. Mol Ther 2007; 15: 1253–1258.
Zhang P, Sun B, Osada T, Rodriguiz R, Yang XY, Luo X et al. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine Pompe disease. Hum Gene Ther 2012; 23: 460–472.
Kosuga M, Takahashi S, Sasaki K, Li XK, Fujino M, Hamada H et al. Adenovirus-mediated gene therapy for mucopolysaccharidosis VII: involvement of cross-correction in wide-spread distribution of the gene products and long-term effects of CTLA-4Ig coexpression. Mol Ther 2000; 1: 406–413.
Nietupski JB, Hurlbut GD, Ziegler RJ, Chu Q, Hodges BL, Ashe KM et al. Systemic administration of AAV8-α-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression. Mol Ther 2011; 19: 1999–2011.
Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC-H, Waterson J et al. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 2006; 149: 89–97.
Lidove O, West ML, Pintos-Morell G, Reisin R, Nicholls K, Figuera LE et al. Effects of enzyme replacement therapy in Fabry disease—a comprehensive review of the medical literature. Genet Med 2010; 12: 668–679.
Banugaria SG, Prater SN, Ng Y-K, Kobori JA, Finkel RS, Ladda RL et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med 2011; 13: 729–736.
Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010; 99: 26–33.
Banugaria SG, Patel TT, Mackey J, Das S, Amalfitano A, Rosenberg AS et al. Persistence of high sustained antibodies to enzyme replacement therapy despite extensive immunomodulatory therapy in an infant with Pompe disease: need for agents to target antibody-secreting plasma cells. Mol Genet Metab 2012; 105: 677–680.
Palmer D, Ng P . Improved system for helper-dependent adenoviral vector production. Mol Ther 2003; 8: 846–852.
Brunetti-Pierri N, Ng T, Iannitti D, Cioffi W, Stapleton G, Law M et al. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum Gene Ther 2013; 24: 761–765.
Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, Zuo Y et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther 2009; 17: 327–333.
Brunetti-Pierri N, Liou A, Patel P, Palmer D, Grove N, Finegold M et al. Balloon catheter delivery of helper-dependent adenoviral vector results in sustained, therapeutic hFIX expression in rhesus macaques. Mol Ther 2012; 20: 1863–1870.
Ponder KP, Wang B, Wang P, Ma X, Herati R, Wang B et al. Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-Ig. Mol Ther 2006; 14: 5–13.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347.
Han S, Li S, Brooks ED, Masat E, Leborgne C, Banugaria S et al. Enhanced efficacy from gene therapy in Pompe disease using coreceptor blockade. Hum Gene Ther 2015; 26: 26–35.
Berrier KL, Kazi ZB, Prater SN, Bali DS, Goldstein J, Stefanescu MC et al. CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy. Genet Med 2015; 17: 912–918.
Morral N, O’Neal WK, Rice K, Leland MM, Piedra PA, Aguilar-Córdova E et al. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther 2002; 13: 143–154.
Amalfitano A, Parks RJ . Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr Gene Ther 2002; 2: 111–133.
Seregin SS, Amalfitano A . Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin Biol Ther 2009; 9: 1521–1531.
Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol 2008; 181: 2134–2144.
Amalfitano A . Utilization of adenovirus vectors for multiple gene transfer applications. Methods 2004; 33: 173–178.
Rastall DPW, Aldhamen YA, Seregin SS, Godbehere S, Amalfitano A . ERAP1 functions override the intrinsic selection of specific antigens as immunodominant peptides, thereby altering the potency of antigen-specific cytolytic and effector memory T-cell responses. Int Immunol 2014; 26: 685–695.
Amalfitano A, Begy CR, Chamberlain JS . Improved adenovirus packaging cell lines to support the growth of replication-defective gene-delivery vectors. Proc Natl Acad Sci USA 1996; 93: 3352–3356.
Aldhamen YA, Seregin SS, Rastall DPW, Aylsworth CF, Pepelyayeva Y, Busuito CJ et al. Endoplasmic reticulum aminopeptidase-1 functions regulate key aspects of the innate immune response. PLoS One 2013; 8: e69539.
Zhang H, Kallwass H, Young SP, Carr C, Dai J, Kishnani PS et al. Comparison of maltose and acarbose as inhibitors of maltase-glucoamylase activity in assaying acid alpha-glucosidase activity in dried blood spots for the diagnosis of infantile Pompe disease. Genet Med 2006; 8: 302–306.
Seregin SS, Aldhamen YA, Rastall DPW, Godbehere S, Amalfitano A . Adenovirus-based vaccination against Clostridium difficile toxin A allows for rapid humoral immunity and complete protection from toxin A lethal challenge in mice. Vaccine 2012; 30: 1492–1501.
Acknowledgements
We thank Michigan State University Laboratory Animal support facility for their assistance in the humane care and maintenance of the mice utilized in this work. We thank Southwest National Primate Research Center for their assistance in the humane care and maintenance of the primates utilized in this work. This work was supported by the Acid Maltase Deficiency Association, the MSU Foundation and the Osteopathic Heritage Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Rastall, D., Seregin, S., Aldhamen, Y. et al. Long-term, high-level hepatic secretion of acid α-glucosidase for Pompe disease achieved in non-human primates using helper-dependent adenovirus. Gene Ther 23, 743–752 (2016). https://doi.org/10.1038/gt.2016.53
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.53
This article is cited by
-
Splice modulating antisense oligonucleotides restore some acid-alpha-glucosidase activity in cells derived from patients with late-onset Pompe disease
Scientific Reports (2020)
-
AAV-mediated transcription factor EB (TFEB) gene delivery ameliorates muscle pathology and function in the murine model of Pompe Disease
Scientific Reports (2017)
-
Gene therapy for monogenic liver diseases: clinical successes, current challenges and future prospects
Journal of Inherited Metabolic Disease (2017)
-
Gene therapy with helper-dependent adenoviral vectors: lessons from studies in large animal models
Virus Genes (2017)
-
Current and Future Treatments for Lysosomal Storage Disorders
Current Treatment Options in Neurology (2017)