Abstract
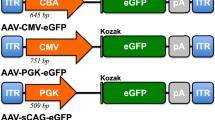
The cat is emerging as a promising large animal model for preclinical testing of retinal dystrophy therapies, for example, by gene therapy. However, there is a paucity of studies investigating viral vector gene transfer to the feline retina. We therefore sought to study the tropism of recombinant adeno-associated viral (rAAV) vectors for the feline outer retina. We delivered four rAAV serotypes: rAAV2/2, rAAV2/5, rAAV2/8 and rAAV2/9, each expressing green fluorescent protein (GFP) under the control of a cytomegalovirus promoter, to the subretinal space in cats and, for comparison, mice. Cats were monitored for gene expression by in vivo imaging and cellular tropism was determined using immunohistochemistry. In cats, rAAV2/2, rAAV2/8 and rAAV2/9 vectors induced faster and stronger GFP expression than rAAV2/5 and all vectors transduced the retinal pigment epithelium (RPE) and photoreceptors. Unlike in mice, cone photoreceptors in the cat retina were more efficiently transduced than rod photoreceptors. In mice, rAAV2/2 only transduced the RPE whereas the other vectors also transduced rods and cones. These results highlight species differences in cellular tropism of rAAV vectors in the outer retina. We conclude that rAAV serotypes are suitable for use for retinal gene therapy in feline models, particularly when cone photoreceptors are the target cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stone EM . Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol 2007; 144: 791–811.
Menotti-Raymond M, David VA, Schaffer AA, Stephens R, Wells D, Kumar-Singh R et al. Mutation in CEP290 discovered for cat model of human retinal degeneration. J Hered 2007; 98: 211–220.
Menotti-Raymond M, Deckman KH, David V, Myrkalo J, O'Brien SJ, Narfstrom K . Mutation discovered in a feline model of human congenital retinal blinding disease. Invest Ophthalmol Vis Sci 2010; 51: 2852–2859.
Furukawa T, Morrow EM, Cepko CL . Crx a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 1997; 91: 531–541.
Linberg KA, Lewis GP, Shaaw C, Rex TS, Fisher SK . Distribution of S- and M-cones in normal and experimentally detached cat retina. Journal Comp Neurol 2001; 430: 343–356.
Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV et al. Gene therapy restores vision in a canine model of childhood blindness. Nat genet 2001; 28: 92–95.
Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci USA 2012; 109: 2132–2137.
Rah H, Maggs DJ, Blankenship TN, Narfström K, Lyons LA . Early-onset autosomal recessive, progressive retinal atrophy in Persian cats. Invest Ophthalmol Vis Sci 2005; 46: 1742–1747.
Buch PK, Bainbridge JW, Ali RR . AAV-mediated gene therapy for retinal disorders: from mouse to man. Gene Therapy 2008; 15: 849–857.
Ho TT, Maguire AM, Aguirre GD, Surace EM, Anand V, Zeng Y et al. Phenotypic rescue after adeno-associated virus-mediated delivery of 4-sulfatase to the retinal pigment epithelium of feline mucopolysaccharidosis VI. J Gene Med 2002; 4: 613–621.
Bainbridge JW, Mistry A, Schlichtenbrede FC, Smith A, Broderick C, De Alwis M et al. Stable rAAV-mediated transduction of rod and cone photoreceptors in the canine retina. Gene Therapy 2003; 10: 1336–1344.
Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M et al. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol 2007; 81: 11372–11380.
Natkunarajah M, Trittibach P, McIntosh J, Duran Y, Barker SE, Smith AJ et al. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Therapy 2008; 15: 463–467.
Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM . Novel AAV serotypes for improved ocular gene transfer. J Gene Med 2008; 10: 375–382.
Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med 2011; 3: 88ra54.
Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 2002; 76: 791–801.
Mowat FM, Gornik KR, Dinculescu A, Boye SL, Hauswirth WW, Petersen-Jones SM et al. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Therapy 2013; 21: 96–105.
Beltran WA . The use of canine models of inherited retinal degeneration to test novel therapeutic approaches. Vet Ophthalmol 2009; 12: 192–204.
Bainbridge JW, Mistry A, Schlichtenbrede FC, Smith A, Broderick C, De Alwis M et al. Stable rAAV-mediated transduction of rod and cone photoreceptors in the canine retina. Gene Therapy 2003; 10: 1336–1344.
Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther 2005; 12: 1072–1082.
Stieger K, Colle MA, Dubreil L, Mendes-Madeira A, Weber M, Le Meur G et al. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther 2008; 16: 916–923.
Vandenberghe LH, Bell P, Maguire AM, Xiao R, Hopkins TB, Grant R et al. AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS One 2013; 8: e53463.
Mussolino C, della Corte M, Rossi S, Viola F, Di Vicino U, Marrocco E et al. AAV-mediated photoreceptor transduction of the pig cone-enriched retina. Gene Therapy 2011; 18: 637–645.
Beltran WA, Boye SL, Boye SE, Chiodo VA, Lewin AS, Hauswirth WW et al. rAAV2/5 gene-targeting to rods:dose-dependent efficiency and complications associated with different promoters. Gene Therapy 2010; 17: 1162–1174.
Gao G, Vandenberghe LH, Wilson JM . New recombinant serotypes of AAV vectors. Curr Gene Ther 2005; 5: 285–297.
Dudus L, Anand V, Acland GM, Chen SJ, Wilson JM, Fisher KJ et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res 1999; 39: 2545–2553.
Mancuso K, Hendrickson AE, Connor Jr TB, Mauck MC, Kinsella JJ, Hauswirth WW et al. Recombinant adeno-associated virus targets passenger gene expression to cones in primate retina. J Opt Soc Am A Opt Image Sci Vis 2007; 24: 1411–1416.
Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010; 21: 704–712.
Anand V, Duffy B, Yang Z, Dejneka NS, Maguire AM, Bennett J . A deviant immune response to viral proteins and transgene product is generated on subretinal administration of adenovirus and adeno-associated virus. Mol Ther 2002; 5: 125–132.
Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis 2008; 14: 1760–1769.
Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P et al. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol 2000; 74: 2420–2425.
Halbert CL, Standaert TA, Wilson CB, Miller AD . Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol 1998; 72: 9795–9805.
Kay MA, Meuse L, Gown AM, Linsley P, Hollenbaugh D, Aruffo A et al. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA 1997; 94: 4686–4691.
Petersen-Jones SM, Bartoe JT, Fischer AJ, Scott M, Boye SL, Chiodo V et al. AAV retinal transduction in a large animal model species: comparison of a self-complementary AAV2/5 with a single-stranded AAV2/5 vector. Mol Vis 2009; 15: 1835–1842.
Tan MH, Smith AJ, Pawlyk B, Xu X, Liu X, Bainbridge JB et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum Mol Genet 2009; 18: 2099–2114.
Mowat FM, Breuwer AR, Bartoe JT, Annear MJ, Zhang Z, Smith AJ et al. RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Therapy 2013; 20: 545–555.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet 2000; 24: 257–261.
Carpentier G, Henault E . Protein array analyzer for ImageJ. In: Tudor H (ed.), Proceedings of the ImageJ User and Developer Conference. Centre de Recherche Public: Belvaux, Luxembourg, 2010, pp 238–240.
Li A, Zhu X, Brown B, Craft CM . Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest Ohthalmol Vis Sci 2003; 44: 996–1007.
Acknowledgements
Funding for this study was provided by the Grousbeck Family Foundation, the Hal and Jean Glassen Memorial Foundation, the Myers Dunlap Endowment (to SMP-J) and research grants from the National Institutes of Health (8DP1EY023177 and 1R24EY019861 to JB, and T32OD011167 to Michigan State University), Foundation Fighting Blindness, and Research to Prevent Blindness. We would like to thank Janice Querubin and Lisa Allen for their assistance and animal care, and Cheryl Craft for providing the cone arrestin antibody.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Minella, A., Mowat, F., Willett, K. et al. Differential targeting of feline photoreceptors by recombinant adeno-associated viral vectors: implications for preclinical gene therapy trials. Gene Ther 21, 913–920 (2014). https://doi.org/10.1038/gt.2014.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.65