Abstract
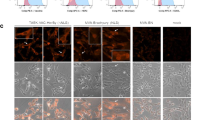
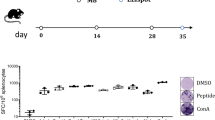
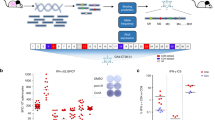
For successful clinical tumor immunotherapy outcomes, strong immune responses against tumor antigens must be generated. Cell-based vaccines compromise one strategy with which to induce appropriate strong immune responses. Previously, we established a natural killer T-cell (NKT) ligand-loaded, adenoviral vector-transduced B-cell-based anticancer cellular vaccine. To enhance tumor antigen delivery to B cells, we established a modified adenoviral vector (Ad-k35) that encoded a truncated form of the breast cancer antigen Her2/neu (Ad-k35HM) in which fiber structure was substituted with adenovirus serotype 35. We observed increased tumor antigen expression with Ad-k35HM in both human and murine B cells. In addition, an Ad-k35HM-transduced B-cell vaccine elicited strong antigen-specific cellular and humoral immune responses that were further enhanced with the additional loading of soluble NKT ligand KBC009. An Ad-k35HM-transduced, KBC009-loaded B-cell vaccine efficiently suppressed the in vivo growth of established tumors in a mouse model. Moreover, the vaccine elicited human leukocyte antigen (HLA)-A2 epitope-specific cytotoxic T-cell responses in B6.Cg (CB)-Tg (HLA-A/H2-D) 2Enge/Jat mice. These findings indicated that the Ad-k35 could be appropriate for the preclinical and clinical development of B-cell-based anticancer immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rosenberg SA, Yang JC, Restifo NP . Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–915.
Schreurs MW, Eggert AA, de Boer AJ, Figdor CG, Adema GJ . Generation and functional characterization of mouse monocyte-derived dendritic cells. Eur J Immunol 1999; 29: 2835–2841.
Elkord E, Williams PE, Kynaston H, Rowbottom AW . Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology 2005; 114: 204–212.
Gilboa E . DC-based cancer vaccines. J Clin Invest 2007; 117: 1195–1203.
Palucka K, Banchereau J . Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12: 265–277.
Chung Y, Kim BS, Kim YJ, Ko HJ, Ko SY, Kim DH et al. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res 2006; 66: 6843–6850.
Kim YJ, Ko HJ, Kim YS, Kim DH, Kang S, Kim JM et al. Alpha-Galactosylceramide-loaded, antigen-expressing B cells prime a wide spectrum of antitumor immunity. Int J Cancer 2008; 122: 2774–2783.
Schultze JL, Grabbe S, von Bergwelt-Baildon MS . DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol 2004; 25: 659–664.
Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest 1997; 100: 2757–2765.
Kim YJ, Han SH, Kang HW, Lee JM, Kim YS, Seo JH et al. NKT ligand-loaded, antigen-expressing B cells function as long-lasting antigen presenting cells in vivo. Cell Immunol 2011; 270: 135–144.
Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol 2008; 181: 2134–2144.
Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I . A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol 1996; 70: 6497–6501.
Xiang ZQ, Yang Y, Wilson JM, Ertl HC . A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 1996; 219: 220–227.
Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002; 415: 331–335.
Tan Y, Hackett NR, Boyer JL, Crystal RG . Protective immunity evoked against anthrax lethal toxin after a single intramuscular administration of an adenovirus-based vaccine encoding humanized protective antigen. Human Gene Ther 2003; 14: 1673–1682.
Shiver JW, Emini EA . Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med 2004; 55: 355–372.
Lasaro MO, Ertl HC . New insights on adenovirus as vaccine vectors. Mol Ther 2009; 17: 1333–1339.
Arnberg N . Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol 2009; 19: 165–178.
Kim YS, Kim YJ, Lee JM, Han SH, Ko HJ, Park HJ et al. CD40-targeted recombinant adenovirus significantly enhances the efficacy of antitumor vaccines based on dendritic cells and B cells. Human Gene Ther 2010; 21: 1697–1706.
Brouwer E, Havenga MJ, Ophorst O, de Leeuw B, Gijsbers L, Gillissen G et al. Human adenovirus type 35 vector for gene therapy of brain cancer: improved transduction and bypass of pre-existing anti-vector immunity in cancer patients. Cancer Gene Ther 2007; 14: 211–219.
McVey D, Zuber M, Ettyreddy D, Reiter CD, Brough DE, Nabel GJ et al. Characterization of human adenovirus 35 and derivation of complex vectors. Virol J 2010; 7: 276.
Wu C, Lei X, Wang J, Hung T . Generation of a replication-deficient recombinant human adenovirus type 35 vector using bacteria-mediated homologous recombination. J Virological Methods 2011; 177: 55–63.
Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 2007; 81: 4654–4663.
Wang H, Liu Y, Li ZY, Fan X, Hemminki A, Lieber A . A recombinant adenovirus type 35 fiber knob protein sensitizes lymphoma cells to rituximab therapy. Blood 2010; 115: 592–600.
Adams WC, Gujer C, McInerney G, Gall JG, Petrovas C, Karlsson Hedestam GB et al. Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc Natl Acad Sci USA 2011; 108: 7499–7504.
Gaggar A, Shayakhmetov DM, Lieber A . CD46 is a cellular receptor for group B adenoviruses. Nat Med 2003; 9: 1408–1412.
Kanerva A, Hemminki A . Modified adenoviruses for cancer gene therapy. Int J Cancer 2004; 110: 475–480.
Kang E, Yun CO . Current advances in adenovirus nanocomplexes: more specificity and less immunogenicity. BMB Rep 2010; 43: 781–788.
Choi JW, Lee JS, Kim SW, Yun CO . Evolution of oncolytic adenovirus for cancer treatment. Adv Drug Delivery Rev 2012; 64: 720–729.
Lee JS, Hur MW, Lee SK, Choi WI, Kwon YG, Yun CO . A novel sLRP6E1E2 inhibits canonical Wnt signaling, epithelial-to-mesenchymal transition, and induces mitochondria-dependent apoptosis in lung cancer. PloS One 2012; 7: e36520.
Yu L, Shimozato O, Li Q, Kawamura K, Ma G, Namba M et al. Adenovirus type 5 substituted with type 11 or 35 fiber structure increases its infectivity to human cells enabling dual gene transfer in CD46-dependent and -independent manners. Anticancer Res 2007; 27: 2311–2316.
Lee YS, Lee KA, Lee JY, Kang MH, Song YC, Baek DJ et al. An alpha-GalCer analogue with branched acyl chain enhances protective immune responses in a nasal influenza vaccine. Vaccine 2011; 29: 417–425.
Murata K, Lechmann M, Qiao M, Gunji T, Alter HJ, Liang TJ . Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proc Natl Acad Sci USA 2003; 100: 6753–6758.
Krishnan A, Wang Z, Srivastava T, Rawal R, Manchanda P, Diamond DJ et al. A novel approach to evaluate the immunogenicity of viral antigens of clinical importance in HLA transgenic murine models. Immunol Lett 2008; 120: 108–116.
Shao HY, Lin YW, Yu SL, Lin HY, Chitra E, Chang YC et al. Immunoprotectivity of HLA-A2 CTL peptides derived from respiratory syncytial virus fusion protein in HLA-A2 transgenic mouse. PloS One 2011; 6: e25500.
Tan WG, Jin HT, West EE, Penaloza-MacMaster P, Wieland A, Zilliox MJ et al. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J Virol 2012; 87: 1359–1372.
Tsujimura A, Shida K, Kitamura M, Nomura M, Takeda J, Tanaka H et al. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J 1998; 330 Pt 1 163–168.
Johnson MJ, Petrovas C, Yamamoto T, Lindsay RW, Lore K, Gall JG et al. Type I IFN induced by adenovirus serotypes 28 and 35 has multiple effects on T cell immunogenicity. J Immunol 2012; 188: 6109–6118.
Deenick EK, Ma CS, Brink R, Tangye SG . Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr Opin Immunol 2011; 23: 111–118.
King C, Sprent J . Emerging cellular networks for regulation of T follicular helper cells. Trends Immunol 2012; 33: 59–65.
Penichet ML, Challita PM, Shin SU, Sampogna SL, Rosenblatt JD, Morrison SL . In vivo properties of three human HER2/neu-expressing murine cell lines in immunocompetent mice. Lab Animal Sci 1999; 49: 179–188.
Peng S, Trimble C, He L, Tsai YC, Lin CT, Boyd DA et al. Characterization of HLA-A2-restricted HPV-16 E7-specific CD8(+) T-cell immune responses induced by DNA vaccines in HLA-A2 transgenic mice. Gene Therapy 2006; 13: 67–77.
Kim J, Kim JH, Choi KJ, Kim PH, Yun CO . E1A- and E1B-Double mutant replicating adenovirus elicits enhanced oncolytic and antitumor effects. Human Gene Ther 2007; 18: 773–786.
Lee YS, Lee KA, Yoon HB, Yoo SA, Park YW, Chung Y et al. The Wnt inhibitor secreted frizzled-related protein 1 (sFRP1) promotes human Th17 differentiation. Eur J Immunol 2012; 42: 2564–2573.
Ko HJ, Kim YJ, Kim YS, Kim JM, Ho SH, Jeong JG et al. Immunogenicity and safety profiles of genetic vaccines against human Her-2/neu in cynomolgus monkeys. Gene Therapy 2008; 15: 1351–1360.
Acknowledgements
This work was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare (0720500) and the World Class University Program (Grant R31-2008-000-10103-0) of the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Kim, EK., Seo, HS., Chae, MJ. et al. Enhanced antitumor immunotherapeutic effect of B-cell-based vaccine transduced with modified adenoviral vector containing type 35 fiber structures. Gene Ther 21, 106–114 (2014). https://doi.org/10.1038/gt.2013.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.65
Keywords
This article is cited by
-
HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development
Archivum Immunologiae et Therapiae Experimentalis (2020)
-
Roles of NKT cells in cancer immunotherapy
Archives of Pharmacal Research (2019)
-
IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours
Nature Communications (2017)