Abstract
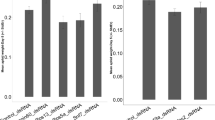
RNA interference (RNAi)-mediated viral inhibition has been used in several organisms for improving viral resistance. In the present study, we reported the use of transgenic RNAi in preventing Bombyx mori nucleopolyhedrovirus (BmNPV) multiplication in the transgenic silkworm B. mori. We targeted the BmNPV immediate-early-1 (ie-1) and late expression factor-1 (lef-1) genes in the transiently transfected BmN cells, in the stable transformed BmN cell line and in the transgenic silkworms. We generated four piggyBac-based vectors containing short double-stranded ie-1 RNA (sdsie-1), short double-stranded lef-1 RNA (sdslef-1), long double-stranded ie-1 RNA (ldsie-1) and both sdsie-1 and sdslef-1 (sds-ie1-lef1) expression cassettes. Strong viral repression was observed in the transiently transfected cells and in the stable transformed BmN cells transfected with sds-ie-1, sdslef-1, ldsie-1 or sds-ie-lef. The decrease of ie-1 mRNA level in the sds-ie1-lef1 transiently transfected cells was most obvious among the cells transfected with different vectors. The inhibitory effect of viral multiplication was decreased in a viral dose-dependent manner; the infection ratio of transfected cells for sds-ie-1, sdslef-1, ldsie-1 and sds-ie-lef decreased by 18.83%, 13.73%, 6.93% and 30.63%, respectively, compared with control cells 5 days after infection. We generated transgenic silkworms using transgenic vector piggyantiIE-lef1-neo with sds-ie1-lef1 expression cassette; the fourth instar larvae of transgenic silkworms of generation G5 exhibited stronger resistance to BmNPV, the mortalities for the transgenic silkworms and control silkworms were 60% and 100%, respectively, at 11 days after inoculation with BmNPV (106 occlusion bodies per ml). These results suggest that double-stranded RNA expression of essential genes of BmNPV is a feasible method for breeding silkworms with a high antiviral capacity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, Martelli GP et al Sixth Report of the International Committee on Taxonomy of Virus. Springer: Vienna, Austria, 1995.
Kool M, Ahrens CH, Goldbach RW, Rohrmann GF, Vlak JM . Identification of genes involved in DNA replication of the Autographa californica baculovirus. Proc Natl Acad Sci USA 1994; 91: 11212–11216.
Lu A, Miller LK . The roles of eighteen baculovirus late expression factor genes in transcription and DNA replication. J Virol 1995; 69: 975–982.
Gomi S, Zhou C, Yih W, Majima K, Maeda S . Deletion analysis of four of eighteen late gene expression factor gene homologues of the baculovirus, BmNPV. Virology 1997; 230: 35–47.
Pathakamuri JA, Theilmann DA . The acidic activation domain of the baculovirus transactivator IE1 contains a virus-specific domain essential for DNA replication. J Virol 2002; 76: 5598–5604.
Mikhailov VS, Rohrmann GF . Baculovirus replication factor LEF-1 is a DNA primase. J Virol 2002; 76: 2287–2297.
Guarino LA, Summers MD . Functional mapping of a trans-activating gene required for expression of a baculovirus delayed-early gene. J Virol 1986; 57: 563–571.
Nissen MS, Friesen PD . Molecular analysis of the transcriptional regulatory region of an early baculovirus gene. J Virol 1989; 63: 493–503.
Carson DD, Summers MD, Guarino LA . Molecular analysis of a baculovirus regulatory gene. Virology 1991; 182: 279–286.
Passarelli A, Miller L . Three baculovirus genes involved in late and very late gene expression: ie-1, ie-n, and lef-2. J Virol 1993; 67: 2149–2158.
Theilmann DA, Stewart S . Identification and characterization of the IE-1 gene of Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology 1991; 180: 492–508.
Choi J, Guarino LA . The baculovirus transactivator IE1 binds to viral enhancer elements in the absence of insect cell factors. J. Virol 1995; 69: 4548–4551.
Guarino LA, Dong W . Expression of an enhancer-binding protein in insect cells transfected with the Autographa californica nuclear polyhedrosis virus IE1 gene. J Virol 1991; 65: 3676–3680.
Rodems SM, Friesen PD . Transcriptional enhancer activity of hr5 requires dual-palindrome half sites that mediate binding of a dimeric form of the baculovirus transregulator IE1. J Virol 1995; 69: 5368–5375.
Jiang L, Wang G, Cheng T, Yang Q, Jin S . Resistance to Bombyx mori nucleopolyhedrovirus via overexpression of an endogenous antiviral gene in transgenic silkworms. Arch Virol 2012; 157: 1323–1328.
Evans JT, Leisy DJ, Rohrmann GF . Characterization of the interaction between the baculovirus replication factors LEF-1 and LEF-2. J. Virol 1997; 71: 3114–3119.
Fire A, Xu S, Montgomery M, Kostas S, Driver S, Mello C . Potent and specific genetic interference by doublestranded RNA in Caenorhabditis elegans. Nature 1998; 391: 806–811.
Catalanotto C, Azzalin G, Macino G, Cogoni C . Gene silencing in worms and fungi. Nature 2000; 404: 245.
Dawe RK . RNA interference, transposons, and the centromere. Plant Cell 2003; 15: 297–301.
Kennerdell JR, Carthew RW . Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 1998; 95: 1017–1026.
Svoboda P, Stein P, Hayashi H, Schultz RM . Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development 2000; 127: 4147–4156.
Quan GX, Kanda T, Tamura T . Induction of the white egg 3 mutant phenotype by injection of the double-stranded RNA of the silkworm white gene. Insect Mol Biol 2002; 11: 217–222.
Fujita K, Sagisaka A . DNA vector-based RNA interference in cell lines derived from Bombyx mori. Biosci Biotechnol Biochem 2009; 73: 2026–2031.
Tanaka H, Fujita K . shRNA expression plasmids generated by a novel method efficiently induce gene-specific knockdown in a silkworm cell line. Mol Biotechnol 2009; 41: 173–179.
Yamaguchi J, Mizoguchi T, Fujiwara H . siRNAs induce efficient RNAi response in Bombyx mori embryos. PLoS One 2011; 6: e25469.
Isobe R, Kojima K, Matsuyama T, Quan GX, Kanda T, Tamura T . Use of RNAi technology to confer enhanced resistance to BmNPV on transgenic silkworms. Arch Virol 2004; 149: 1931–1940.
Kanginakudru S, Royer C, Edupalli SV . Targeting ie-1 gene by RNAi induces baculoviral resistance in lepidopteran cell lines and in transgenic silkworms. Insect Mol Biol 2007; 16: 635–644.
Jiang L, Cheng T, Zhao P, Yang Q, Wang G . Resistance to BmNPV via overexpression of an exogenous gene controlled by an inducible promoter and enhancer in transgenic silkworm, Bombyx mori. PLoS One 2012; 7 (8): e41838.
Subbaiah EV, Royer C, Kanginakudru S, Satyavathi VV, Babu AS, Sivaprasad V et al. Engineering silkworms for resistance to baculovirus through multigene rna interference. Genetics 2013; 193: 63–75.
Hammond SM, Caudy AA, Hannon GJ . Post-transcriptional gene silencing by double-stranded RNA. Nat Rev Gen 2001; 2: 110–119.
Flores-Jasso CF, Valdes VJ, Sampieri A, Valadez-Graham V, Recillas-Targa F, Vaca L . Silencing structural and nonstructural genes in baculovirus by RNA interference. Virus Res 2004; 102: 75–84.
Swevers L, Liu J, Huvenne H, Smagghe G . Search for limiting factors in the RNAi pathway in silkmoth tissues and the Bm5 cell line: the RNA binding proteins R2D2 and translin. PLoS One 2011; 6: e20250.
Liu Y, Ye X, Jiang F, Liang C, Chen D . C3PO, an endoribonuclease that promotes RNAi by facilitating RISC activation. Science 2009; 325: 750–753.
Zhou W, Wang C, Liu B, Cao G, Xue R, Shen W et al. The elementary transgene research of silkworm cells mediated by transposon piggyBac. Acta Sericol Sin 2007; 33: 30–35.
Tamura T, Thibert C, Royer C . Germline transformation of the si1kworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol 2000; 18: 81–84.
Zhao Y, Li X, Cao G, Xue R, Gong C . Expression of hIGF-I in silk glands of transgenic silkworms and in transformed silkworm cells. Sci China C 2009; 52: 1131–1139.
Acknowledgements
We gratefully acknowledge the financial support of the National Basic Research Program of China (Program 973, 2012CB114600), the National Natural Science Foundation of China (31072085, 31272500), the Specialized Research Fund for the Doctoral Program of Higher Education (20113201130002), the Key Fostering Project for Application Research of Soochow University (Q3134991) and a project funded by the Priority Academic Program of Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, P., Wang, J., Lu, Y. et al. Resistance of transgenic silkworm to BmNPV could be improved by silencing ie-1 and lef-1 genes. Gene Ther 21, 81–88 (2014). https://doi.org/10.1038/gt.2013.60
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.60
Keywords
This article is cited by
-
Recombinase-aided amplification coupled with lateral flow dipstick for efficient and accurate detection of Bombyx mori nucleopolyhedrovirus
Folia Microbiologica (2023)
-
Targeting genes involved in nucleopolyhedrovirus DNA multiplication through RNA interference technology to induce resistance against the virus in silkworms
Molecular Biology Reports (2020)
-
Modulation of NPV gene expression pattern and retention of RNAi- based antiviral activity in inbred transgenic silkworm
International Journal of Tropical Insect Science (2020)
-
Construction of a One-Vector Multiplex CRISPR/Cas9 Editing System to Inhibit Nucleopolyhedrovirus Replication in Silkworms
Virologica Sinica (2019)
-
Establishment of a baculovirus-inducible CRISPR/Cas9 system for antiviral research in transgenic silkworms
Applied Microbiology and Biotechnology (2018)