Abstract
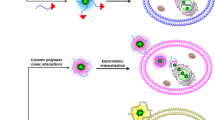
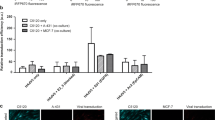
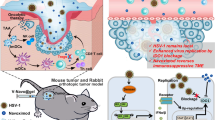
Adenoviruses (Ad) have been investigated for their efficacy in reducing primary tumors after local intratumoral administration. Despite high Ad concentrations and repetitive administration, the therapeutic efficacy of Ad has been limited because of rapid dissemination of the Ad into the surrounding normal tissues and short maintenance of Ad biological activity in vivo. To maximize the therapeutic potential of Ad-mediated gene therapeutics, we investigated the efficacy of local, sustained Ad delivery, using an injectable alginate gel matrix system. The biological activity of Ad loaded in alginate gel was prolonged compared with naked Ad, as evidenced by the high green fluorescent protein gene transduction efficiency over an extended time period. Moreover, oncolytic Ad encapsulated in alginate gel elicited 1.9- to 2.4-fold greater antitumor activity than naked Ad in both C33A and U343 human tumor xenograft models. Histological and quantitative PCR analysis confirmed that the oncolytic Ad/alginate gel matrix system significantly increased preferential replication and dissemination of oncolytic Ad in a larger area of tumor tissue in vivo. Taken together, these results show that local sustained delivery of oncolytic Ad in alginate gel augments therapeutic effect through selective infection of tumor cells, sustained release and prolonged maintenance of Ad activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kim E, Kim JH, Shin HY, Lee H, Yang JM, Kim J et al. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum Gene Ther 2003; 14: 1415–1428.
Kim J, Cho JY, Kim JH, Jung KC, Yun CO . Evaluation of E1B gene-attenuated replicating adenoviruses for cancer gene therapy. Cancer Gene Ther 2002; 9: 725–736.
Kim J, Kim JH, Choi KJ, Kim PH, Yun CO . E1A- and E1B-Double mutant replicating adenovirus elicits enhanced oncolytic and antitumor effects. Hum Gene Ther 2007; 18: 773–786.
Kwon OJ, Kim PH, Huyn S, Wu L, Kim M, Yun CO . A hypoxia- and {alpha}-fetoprotein-dependent oncolytic adenovirus exhibits specific killing of hepatocellular carcinomas. Clin Cancer Res 2010; 16: 6071–6082.
Kirn D . Replication-selective oncolytic adenoviruses: virotherapy aimed at genetic targets in cancer. Oncogene 2000; 19: 6660–6669.
Pesonen S, Diaconu I, Cerullo V, Escutenaire S, Raki M, Kangasniemi L et al. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int J Cancer 2012; 130: 1937–1947.
Ramachandra M, Rahman A, Zou A, Vaillancourt M, Howe JA, Antelman D et al. Re-engineering adenovirus regulatory pathways to enhance oncolytic specificity and efficacy. Nat Biotechnol 2001; 19: 1035–1041.
Alemany R, Balague C, Curiel DT . Replicative adenoviruses for cancer therapy. Nat Biotechnol 2000; 18: 723–727.
Curiel DT, Rancourt C . Conditionally replicative adenoviruses for cancer therapy. Adv Drug Deliv Rev 1997; 27: 67–81.
Choi IK, Lee YS, Yoo JY, Yoon AR, Kim H, Kim DS et al. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther 2010; 17: 190–201.
Yun CO, Kim E, Koo T, Kim H, Lee YS, Kim JH . ADP-overexpressing adenovirus elicits enhanced cytopathic effect by induction of apoptosis. Cancer Gene Ther 2005; 12: 61–71.
Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther 2006; 13: 1010–1020.
Lee YS, Kim JH, Choi KJ, Choi IK, Kim H, Cho S et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin Cancer Res 2006; 12: 5859–5868.
Huang JH, Zhang SN, Choi KJ, Choi IK, Kim JH, Lee MG et al. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol Ther 2010; 18: 264–274.
Heise C, Kirn DH . Replication-selective adenoviruses as oncolytic agents. J Clin Invest 2000; 105: 847–851.
Hoti N, Chowdhury WH, Mustafa S, Ribas J, Castanares M, Johnson T et al. Armoring CRAds with p21/Waf-1 shRNAs: the next generation of oncolytic adenoviruses. Cancer Gene Ther 2010; 17: 585–597.
Leja J, Nilsson B, Yu D, Gustafson E, Akerstrom G, Oberg K et al. Double-detargeted oncolytic adenovirus shows replication arrest in liver cells and retains neuroendocrine cell killing ability. PLoS One 2010; 5: e8916.
Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer JM, Shashkova EV et al. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther 2009; 16: 644–654.
Barton KN, Paielli D, Zhang Y, Koul S, Brown SL, Lu M et al. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol Ther 2006; 13: 347–356.
Choi IK, Lee JS, Zhang SN, Park J, Lee KM, Sonn CH et al. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rbeta(2) or IL-18Ralpha. Gene Ther 2011; 18: 898–909.
Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO . Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst 2006; 98: 1482–1493.
Yoo JY, Kim JH, Kim J, Huang JH, Zhang SN, Kang YA et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther 2008; 15: 635–651.
Yoo JY, Kim JH, Kwon YG, Kim EC, Kim NK, Choi HJ et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther 2007; 15: 295–302.
Evans RK, Nawrocki DK, Isopi LA, Williams DM, Casimiro DR, Chin S et al. Development of stable liquid formulations for adenovirus-based vaccines. J Pharm Sci 2004; 93: 2458–2475.
Pei DS, Zheng JN . Oncolytic adenoviruses expressing interleukin: a novel antitumour approach. Expert Opin Biol Ther 2010; 10: 917–926.
Yang Z, Zhang Q, Xu K, Shan J, Shen J, Liu L et al. Combined therapy with cytokine-induced killer cells and oncolytic adenovirus expressing IL-12 induce enhanced antitumor activity in liver tumor model. PLoS One 2012; 7: e44802.
Li JL, Liu HL, Zhang XR, Xu JP, Hu WK, Liang M et al. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther 2009; 16: 376–382.
Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med 2000; 6: 879–885.
Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res 2000; 60: 6359–6366.
Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM et al. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther 2001; 3: 28–35.
Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Hum Gene Ther 2003; 14: 425–433.
Li HL, Li S, Shao JY, Lin XB, Cao Y, Jiang WQ et al. Pharmacokinetic and pharmacodynamic study of intratumoral injection of an adenovirus encoding endostatin in patients with advanced tumors. Gene Ther 2008; 15: 247–256.
Hatefi A, Cappello J, Ghandehari H . Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm Res 2007; 24: 773–779.
Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol 2003; 21: 2508–2518.
Amento EP, Bhan AK, McCullagh KG, Krane SM . Influences of gamma interferon on synovial fibroblast-like cells. Ia induction and inhibition of collagen synthesis. J Clin Invest 1985; 76: 837–848.
Seth P, Wang ZG, Pister A, Zafar MB, Kim S, Guise T et al. Development of oncolytic adenovirus armed with a fusion of soluble transforming growth factor-beta receptor II and human immunoglobulin Fc for breast cancer therapy. Hum Gene Ther 2006; 17: 1152–1160.
Gugala Z, Davis AR, Fouletier-Dilling CM, Gannon FH, Lindsey RW, Olmsted-Davis EA . Adenovirus BMP2-induced osteogenesis in combination with collagen carriers. Biomaterials 2007; 28: 4469–4479.
Levy RJ, Song C, Tallapragada S, DeFelice S, Hinson JT, Vyavahare N et al. Localized adenovirus gene delivery using antiviral IgG complexation. Gene Ther 2001; 8: 659–667.
Shin S, Shea LD . Lentivirus immobilization to nanoparticles for enhanced and localized delivery from hydrogels. Mol Ther 2010; 18: 700–706.
Okino H, Manabe T, Tanaka M, Matsuda T . Novel therapeutic strategy for prevention of malignant tumor recurrence after surgery: Local delivery and prolonged release of adenovirus immobilized in photocured, tissue-adhesive gelatinous matrix. J Biomed Mater Res A 2003; 66: 643–651.
Greish K, Araki K, Li D, O’Malley BW, Dandu R, Frandsen J et al. Silk-elastinlike protein polymer hydrogels for localized adenoviral gene therapy of head and neck tumors. Biomacromolecules 2009; 10: 2183–2188.
Greish K, Frandsen J, Scharff S, Gustafson J, Cappello J, Li D et al. Silk-elastinlike protein polymers improve the efficacy of adenovirus thymidine kinase enzyme prodrug therapy of head and neck tumors. J Gene Med 2010; 12: 572–579.
George M, Abraham TE . Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan--a review. J Control Release 2006; 114: 1–14.
Koutsopoulos S, Unsworth LD, Nagai Y, Zhang S . Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci USA 2009; 106: 4623–4628.
Chan AW, Neufeld RJ . Tuneable semi-synthetic network alginate for absorptive encapsulation and controlled release of protein therapeutics. Biomaterials 2010; 31: 9040–9047.
Hou T, Xu J, Li Q, Feng J, Zen L . In vitro evaluation of a fibrin gel antibiotic delivery system containing mesenchymal stem cells and vancomycin alginate beads for treating bone infections and facilitating bone formation. Tissue Eng Part A 2008; 14: 1173–1182.
Driesse MJ, Esandi MC, Kros JM, Avezaat CJ, Vecht C, Zurcher C et al. Intra-CSF administered recombinant adenovirus causes an immune response-mediated toxicity. Gene Ther 2000; 7: 1401–1409.
Davis JJ, Fang B . Oncolytic virotherapy for cancer treatment: challenges and solutions. J Gene Med 2005; 7: 1380–1389.
Muruve DA . The innate immune response to adenovirus vectors. Hum Gene Ther 2004; 15: 1157–1166.
Muruve DA, Barnes MJ, Stillman IE, Libermann TA . Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther 1999; 10: 965–976.
Ruzek MC, Kavanagh BF, Scaria A, Richards SM, Garman RD . Adenoviral vectors stimulate murine natural killer cell responses and demonstrate antitumor activities in the absence of transgene expression. Mol Ther 2002; 5: 115–124.
Kalicharran KK, Springthorpe VS, Sattar SA . Studies on the stability of a human adenovirus-rabies recombinant vaccine. Can J Vet Res 1992; 56: 28–33.
Acknowledgements
This research was supported by grants from the Ministry of Knowledge Economy (10030051, to C-OY), the Korea Science and Engineering Foundation (R15-2004-024-02001-0, 2009K001644, 2010-0029220, to C-OY), the Korea Food and Drug Administration (KFDA-10172-332, to C-OY), the Research Fund (WCU 200900000000024, S-WK) of the Ministry of Education, Science and Technology, Korea, and (CA 107070, S-WK) of the National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Choi, JW., Kang, E., Kwon, OJ. et al. Local sustained delivery of oncolytic adenovirus with injectable alginate gel for cancer virotherapy. Gene Ther 20, 880–892 (2013). https://doi.org/10.1038/gt.2013.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.10
Keywords
This article is cited by
-
Overcoming the limitations of locally administered oncolytic virotherapy
BMC Biomedical Engineering (2019)