Abstract
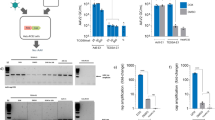
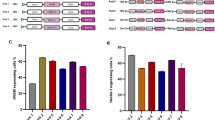
Standard methods for producing high-capacity adenoviral vectors (HC-Ads) are based on co-infection with a helper adenovirus (HV). To avoid HV encapsidation, its packaging signal (Ψ) is flanked by recognition sequences for recombinases expressed in the producing cells. However, accumulation of HV and low yield of HC-Ad are frequently observed, due in part to insufficient recombinase expression. We describe here a novel HV (AdTetCre) in which Ψ is flanked by loxP sites that can be excised by a chimeric MerCreMer recombinase encoded in the same viral genome. Efficient modulation of cleavage was obtained by simultaneous control of MerCreMer expression using a tet-on inducible system, and translocation to the nucleus by 4-hydroxytamoxifen (TAM). Encapsidation of AdTetCre was strongly inhibited by TAM plus doxycicline. Using AdTetCre and 293Cre4 cells for the production of HC-Ads, we found that cellular and virus-encoded recombinases cooperate to minimize HV contamination. The method was highly reproducible and allowed the routine production of different HC-Ads in a medium-scale laboratory setting in adherent cells, with titers >1010 infectious units and <0.1% HV contamination. The residual HVs lacked Ψ and were highly attenuated. We conclude that self-inactivating HVs based on virally encoded recombinases are promising tools for the production of HC-Ads.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Morral N, O’Neal W, Rice K, Leland M, Kaplan J, Piedra PA et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA 1999; 96: 12816–12821.
Alba R, Bosch A, Chillon M . Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Therapy 2005; 12 (Suppl 1): S18–S27.
Brunetti-Pierri N, Ng P . Progress and prospects: gene therapy for genetic diseases with helper-dependent adenoviral vectors. Gene Therapy 2008; 15: 553–560.
Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I . A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol 1996; 70: 6497–6501.
Umana P, Gerdes CA, Stone D, Davis JR, Ward D, Castro MG et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat Biotechnol 2001; 19: 582–585.
Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL . A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA 1996; 93: 13565–13570.
Suzuki M, Cela R, Clarke C, Bertin TK, Mourino S, Lee B . Large-scale production of high-quality helper-dependent adenoviral vectors using adherent cells in cell factories. Hum Gene Ther 2010; 21: 120–126.
Jager L, Hausl MA, Rauschhuber C, Wolf NM, Kay MA, Ehrhardt A . A rapid protocol for construction and production of high-capacity adenoviral vectors. Nat Protoc 2009; 4: 547–564.
Muhammad AK, Puntel M, Candolfi M, Salem A, Yagiz K, Farrokhi C et al. Study of the efficacy, biodistribution, and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin Pharmacol Ther 2010; 88: 204–213.
Puntel M, Muhammad AK, Candolfi M, Salem A, Yagiz K, Farrokhi C et al. A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3 L for glioma therapeutics. J Virol 2010; 84: 6007–6017.
Alba R, Hearing P, Bosch A, Chillon M . Differential amplification of adenovirus vectors by flanking the packaging signal with attB/attP-PhiC31 sequences: implications for helper-dependent adenovirus production. Virology 2007; 367: 51–58.
Palmer D, Ng P . Improved system for helper-dependent adenoviral vector production. Mol Ther 2003; 8: 846–852.
Ng P, Evelegh C, Cummings D, Graham FL . Cre levels limit packaging signal excision efficiency in the Cre/loxP helper-dependent adenoviral vector system. J Virol 2002; 76: 4181–4189.
Zabala M, Wang L, Hernandez-Alcoceba R, Hillen W, Qian C, Prieto J et al. Optimization of the Tet-on system to regulate interleukin 12 expression in the liver for the treatment of hepatic tumors. Cancer Res 2004; 64: 2799–2804.
Verrou C, Zhang Y, Zurn C, Schamel WW, Reth M . Comparison of the tamoxifen regulated chimeric Cre recombinases MerCreMer and CreMer. Biol Chem 1999; 380: 1435–1438.
Crettaz J, Olague C, Vales A, Aurrekoetxea I, Berraondo P, Otano I et al. Characterization of high-capacity adenovirus production by the quantitative real-time polymerase chain reaction: a comparative study of different titration methods. J Gene Med 2008; 10: 1092–1101.
Dormond E, Perrier M, Kamen A . Identification of critical infection parameters to control helper-dependent adenoviral vector production. J Biotechnol 2009; 142: 142–150.
Wang L, Hernandez-Alcoceba R, Shankar V, Zabala M, Kochanek S, Sangro B et al. Prolonged and inducible transgene expression in the liver using gutless adenovirus: a potential therapy for liver cancer. Gastroenterology 2004; 126: 278–289.
Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res 2007; 13: 4677–4685.
Smith GA, Enquist LW . A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc Natl Acad Sci USA 2000; 97: 4873–4878.
Schmid SI, Hearing P . Cellular components interact with adenovirus type 5 minimal DNA packaging domains. J Virol 1998; 72: 6339–6347.
Ostapchuk P, Hearing P . Control of adenovirus packaging. J Cell Biochem 2005; 96: 25–35.
Maeda Y, Kimura E, Uchida Y, Nishida Y, Yamashita S, Arima T et al. Cre/loxP-mediated adenovirus type 5 packaging signal excision demonstrates that core element VI is sufficient for virus packaging. Virology 2003; 309: 330–338.
Yoshimura K, Rosenfeld MA, Seth P, Crystal RG . Adenovirus-mediated augmentation of cell transfection with unmodified plasmid vectors. J Biol Chem 1993; 268: 2300–2303.
Sargent KL, Ng P, Evelegh C, Graham FL, Parks RJ . Development of a size-restricted pIX-deleted helper virus for amplification of helper-dependent adenovirus vectors. Gene Therapy 2004; 11: 504–511.
Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 1998; 9: 1909–1917.
Ng P, Beauchamp C, Evelegh C, Parks R, Graham FL . Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol Ther 2001; 3: 809–815.
Dormond E, Chahal P, Bernier A, Tran R, Perrier M, Kamen A . An efficient process for the purification of helper-dependent adenoviral vector and removal of helper virus by iodixanol ultracentrifugation. J Virol Methods 2010; 165: 83–89.
Chen L, Anton M, Graham FL . Production and characterization of human 293 cell lines expressing the site-specific recombinase Cre. Somat Cell Mol Genet 1996; 22: 477–488.
Qian C, Bilbao R, Bruna O, Prieto J . Induction of sensitivity to ganciclovir in human hepatocellular carcinoma cells by adenovirus-mediated gene transfer of herpes simplex virus thymidine kinase. Hepatology 1995; 22: 118–123.
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B . A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Toietta G, Pastore L, Cerullo V, Finegold M, Beaudet AL, Lee B . Generation of helper-dependent adenoviral vectors by homologous recombination. Mol Ther 2002; 5: 204–210.
Ng P, Parks RJ, Graham FL . Preparation of helper-dependent adenoviral vectors. Methods Mol Med 2002; 69: 371–388.
Dormond E, Meneses-Acosta A, Jacob D, Durocher Y, Gilbert R, Perrier M et al. An efficient and scalable process for helper-dependent adenoviral vector production using polyethylenimine-adenofection. Biotechnol Bioeng 2009; 102: 800–810.
Hernandez-Alcoceba R, Pihalja M, Wicha MS, Clarke MF . A novel, conditionally replicative adenovirus for the treatment of breast cancer that allows controlled replication of E1a-deleted adenoviral vectors. Hum Gene Ther 2000; 11: 2009–2024.
Acknowledgements
This work was funded in part by Fundacion Ramon Areces; Fundacion MMA; Grants SAF2009-11324, BFU2007-60228; BFU2010-16382 from the Spanish Department of Science; and the UTE project CIMA. MGA was supported by a Torres Quevedo contract from the Spanish Department of Science. The technical support of Marian Fernandez Estevez is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Gonzalez-Aparicio, M., Mauleon, I., Alzuguren, P. et al. Self-inactivating helper virus for the production of high-capacity adenoviral vectors. Gene Ther 18, 1025–1033 (2011). https://doi.org/10.1038/gt.2011.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.58
Keywords
This article is cited by
-
Safety and antitumor effect of oncolytic and helper-dependent adenoviruses expressing interleukin-12 variants in a hamster pancreatic cancer model
Gene Therapy (2015)
-
Helper-dependent adenovirus achieve more efficient and persistent liver transgene expression in non-human primates under immunosuppression
Gene Therapy (2015)
-
Impact of adenovirus life cycle progression on the generation of canine helper-dependent vectors
Gene Therapy (2015)
-
Canine helper-dependent vectors production: implications of Cre activity and co-infection on adenovirus propagation
Scientific Reports (2015)