Abstract
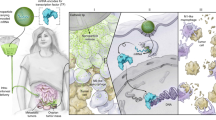
We previously identified the mouse and human Glipr1 and GLIPR1/RTVP-1 genes, respectively, as direct p53 targets with proapoptotic activities in various cancer cell lines, including prostate cancer (PCa). Intratumoral injection of an adenoviral vector capable of efficient transduction and expression of Glipr1 (AdGlipr1) yielded promising therapeutic results in an orthotopic, metastatic mouse model of PCa. AdGlipr1-transduced macrophages (Mφ/Glipr1) generated greater surface expression of CD40, CD80 and major histocompatibility complex class II molecules and greater production of interleukin 12 (IL-12) and IL-6 in vitro than control macrophages did. Mechanistic analysis indicated that increased production of IL-12 in Mφ/Glipr1 depends on activation of the p38 signaling cascade. Mφ/Glipr1 injected into orthotopic 178-2BMA tumors in vivo resulted in significantly suppressed prostate tumor growth and spontaneous lung metastases and longer survival relative to those observed in control-treated mice. Furthermore, these preclinical data indicate the generation of systemic natural killer cell activity and tumor-specific cytotoxic T lymphocyte responses. Trafficking studies confirmed that intratumorally injected Mφ/Glipr1 could migrate to draining lymph nodes. Overall, our data suggest that this novel gene-modified cell approach is an effective treatment avenue that induces antitumor immune responses in preclinical studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E . Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512.
Vogelstein B, Kinzler KW . p53 function and dysfunction. Cell 1992; 70: 523–526.
Che M, DeSilvio M, Pollack A, Grignon DJ, Venkatesan VM, Hanks GE et al. Prognostic value of abnormal p53 expression in locally advanced prostate cancer treated with androgen deprivation and radiotherapy: a study based on RTOG 9202. Int J Radiat Oncol Biol Phys 2007; 69: 1117–1123.
Thompson TC, Timme TL, Sehgal I . Oncogenes, growth factors and hormones in prostate cancer. In: Dickson RB, Salomon SD (eds). Hormones and Growth Factors in Development and Neoplasia. John Wiley and Sons: New York, 1998.
Ren C, Li L, Goltsov AA, Timme TL, Tahir SA, Wang J et al. mRTVP-1, a novel p53 target gene with proapoptotic activities. Mol Cell Biol 2002; 22: 3345–3357.
Rich T, Chen P, Furman F, Huynh N, Israel MA . RTVP-1, a novel human gene with sequence similarity to genes of diverse species, is expressed in tumor cell lines of glial but not neuronal origin. Gene 1996; 180: 125–130.
Murphy EV, Zhang Y, Zhu W, Biggs J . The human glioma pathogenesis-related protein is structurally related to plant pathogenesis-related proteins and its gene is expressed specifically in brain tumors. Gene 1995; 159: 131–135.
Gingras MC, Margolin JF . Differential expression of multiple unexpected genes during U937 cell and macrophage differentiation detected by suppressive subtractive hybridization. Exp Hematol 2000; 28: 65–76.
Ren C, Li L, Yang G, Timme TL, Goltsov A, Ren C et al. RTVP-1, a tumor suppressor inactivated by methylation in prostate cancer. Cancer Res 2004; 64: 969–976.
Satoh T, Timme TL, Saika T, Ebara S, Yang G, Wang J et al. Adenoviral vector-mediated mRTVP-1 gene therapy for prostate cancer. Hum Gene Ther 2003; 14: 91–101.
Naruishi K, Timme TL, Kusaka N, Fujita T, Yang G, Goltsov A et al. Adenoviral vector-mediated RTVP-1 gene-modified tumor cell-based vaccine suppresses the development of experimental prostate cancer. Cancer Gene Ther 2006; 13: 658–663.
Wang H, Thompson TC . Gene-modified bone marrow cell therapy for prostate cancer. Gene Therapy 2008; 15: 787–796.
Prill JM, Espenlaub S, Samen U, Engler T, Schmidt E, Vetrini F et al. Modifications of adenovirus Hexon allow for either hepatocyte detargeting or targeting with potential evasion from Kupffer cells. Mol Ther 2011; 19: 83–92.
Satoh T, Saika T, Ebara S, Kusaka N, Timme TL, Yang G et al. Macrophages transduced with an adenoviral vector expressing interleukin 12 suppress tumor growth and metastasis in a preclinical metastatic prostate cancer model. Cancer Res 2003; 63: 7853–7860.
Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, Thompson TC . Reduced infiltration of tumor-associated macrophages in human prostate cancer: association with cancer progression. Cancer Res 2000; 60: 5857–5861.
Gordon S . Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35.
Tsung K, Dolan JP, Tsung YL, Norton JA . Macrophages as effector cells in interleukin 12-induced T cell-dependent tumor rejection. Cancer Res 2002; 62: 5069–5075.
Alleva DG, Askew D, Burger CJ, Elgert KD . Fibrosarcoma-induced increase in macrophage tumor necrosis factor alpha synthesis suppresses T cell responses. J Leukoc Biol 1993; 54: 152–160.
Hildenbrand R, Jansen C, Wolf G, Bohme B, Berger S, von Minckwitz G et al. Transforming growth factor-beta stimulates urokinase expression in tumor-associated macrophages of the breast. Lab Invest 1998; 78: 59–71.
Bonta IL, Ben-Efraim S . Involvement of inflammatory mediators in macrophage antitumor activity. J Leukoc Biol 1993; 54: 613–626.
Oghiso Y, Yamada Y, Ando K, Ishihara H, Shibata Y . Differential induction of prostaglandin E2-dependent and -independent immune suppressor cells by tumor-derived GM-CSF and M-CSF. J Leukoc Biol 1993; 53: 86–92.
Alleva DG, Walker TM, Elgert KD . Induction of macrophage suppressor activity by fibrosarcoma-derived transforming growth factor-beta 1: contrasting effects on resting and activated macrophages. J Leukoc Biol 1995; 57: 919–928.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A . Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549–555.
Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res 2003; 23: 5015–5022.
Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE . Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep 2003; 10: 309–313.
Piras F, Colombari R, Minerba L, Murtas D, Floris C, Maxia C et al. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 2005; 104: 1246–1254.
Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S . Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol 2004; 99: 1667–1674.
Leek RD, Landers RJ, Harris AL, Lewis CE . Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 1999; 79: 991–995.
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 2010; 70: 5728–5739.
Hommes DW, Peppelenbosch MP, van Deventer SJ . Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003; 52: 144–151.
Kim L, Del Rio L, Butcher BA, Mogensen TH, Paludan SR, Flavell RA et al. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J Immunol 2005; 174: 4178–4184.
Salmon RA, Guo X, Teh HS, Schrader JW . The p38 mitogen-activated protein kinases can have opposing roles in the antigen-dependent or endotoxin-stimulated production of IL-12 and IFN-gamma. Eur J Immunol 2001; 31: 3218–3227.
Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ et al. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J 1999; 18: 1845–1857.
Li L, Abdel Fattah E, Cao G, Ren C, Yang G, Goltsov AA et al. Glioma pathogenesis-related protein 1 exerts tumor suppressor activities through proapoptotic reactive oxygen species-c-Jun-NH2 kinase signaling. Cancer Res 2008; 68: 434–443.
Uzgare AR, Isaacs JT . Enhanced redundancy in Akt and mitogen-activated protein kinase-induced survival of malignant versus normal prostate epithelial cells. Cancer Res 2004; 64: 6190–6199.
Eastham JA, Chen SH, Sehgal I, Yang G, Timme TL, Hall SJ et al. Prostate cancer gene therapy: herpes simplex virus thymidine kinase gene transduction followed by ganciclovir in mouse and human prostate cancer models. Hum Gene Ther 1996; 7: 515–523.
Nasu Y, Bangma CH, Hull GW, Lee HM, Hu J, Wang J et al. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Therapy 1999; 6: 338–349.
Spandidos A, Wang X, Wang H, Seed B . PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 2010; 38: D792–D799.
Shaker MR, Yang G, Timme TL, Park SH, Kadmon D, Ren C et al. Dietary 4-HPR suppresses the development of bone metastasis in vivo in a mouse model of prostate cancer progression. Clin Exp Metastasis 2000; 18: 429–438.
Thompson TC, Southgate J, Kitchener G, Land H . Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell 1989; 56: 917–930.
Lee HM, Timme TL, Thompson TC . Resistance to lysis by cytotoxic T cells: a dominant effect in metastatic mouse prostate cancer cells. Cancer Res 2000; 60: 1927–1933.
Acknowledgements
This work was supported by the National Cancer Institute Grant R01 CA50588.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Some of the data included in this paper are relevant to intellectual property, co-invented by one of the authors (TCT), held by Baylor College of Medicine, and licensed to Progression Therapeutics Inc., a private biotechnology start-up.
Rights and permissions
About this article
Cite this article
Tabata, K., Kurosaka, S., Watanabe, M. et al. Tumor growth and metastasis suppression by Glipr1 gene-modified macrophages in a metastatic prostate cancer model. Gene Ther 18, 969–978 (2011). https://doi.org/10.1038/gt.2011.51
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.51
Keywords
This article is cited by
-
Glioma pathogenesis-related protein 1 performs dual functions in tumor cells
Cancer Gene Therapy (2022)
-
GLIPR1-ΔTM synergizes with docetaxel in cell death and suppresses resistance to docetaxel in prostate cancer cells
Molecular Cancer (2015)
-
The latest advances of experimental research on targeted gene therapy for prostate cancer
The Chinese-German Journal of Clinical Oncology (2013)
-
Prostate Cancer and Immunoproteome: Awakening and Reprogramming the Guardian Angels
Archivum Immunologiae et Therapiae Experimentalis (2012)