Abstract
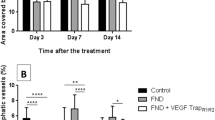
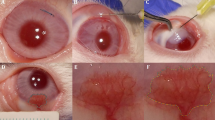
Corneal neovascularization can reduce visual acuity. GA-binding protein (GABP) is a transcription factor that regulates the expression of target genes including vascular endothelial growth factor (VEGF) and roundabout4 (Robo4), which participate in pathologic angiogenesis. We assessed whether intraocular injection of the GABP gene affects the growth of new corneal blood vessels in a mouse ocular neovascularization model. Transfection of human GABPα and GABPβ gene (GABPα/β) into human conjunctival epithelial cells resulted in decreased VEGF and Robo4 expression. Three groups of mice underwent chemical and mechanical denudation of the corneal epithelium. Subsequently, two groups were administered subconjunctival injection of lipoplexes carrying plasmid DNA encoding for human GABPα/β or an empty plasmid DNA at 1-week intervals. The third group served as an experimental control. In vivo delivery of human GABPα/β into mouse neovascularized cornea reduced VEGF and Robo4 gene expression. Biomicroscopic examination showed that, at 1 week after one or two injections, GABPα/β-treated eyes had significantly less neovascularized corneal area than did eyes treated with the empty vector. Histologic examination showed significantly less vascularized area and fewer blood vessels in the GABP-treated group at 1 week after injections. However, these angiosuppressive effects were weakened at 2 weeks after injections. Our results indicate that subconjunctival GABP gene delivery delays corneal neovascularization for up to 2 weeks in a mouse model of deliberate corneal injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kvanta A, Sarman S, Fagerholm P, Seregard S, Steen B . Expression of matrix metalloproteinase-2 and vascular endothelial growth factor in inflammation-associated corneal neovascularization. Exp Eye Res 2000; 70: 419–428.
Chang JH, Gabison EE, Kato T, Azar DT . Corneal neovascualrization. Curr Opin Ophthalmol 2001; 12: 242–249.
Gabison E, Chang JH, Hernandez-Quintela E, Javier J, Lu PC, Ye H et al. Anti-angiogenic role of angiostatin during corneal wound healing. Exp Eye Res 2004; 78: 579–589.
Folkman J . Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31.
Lai YK, Shen WY, Brankov M, Lai CM, Constable IJ, Rakoczy PE . Potential long-term inhibition of ocular neovascularisation by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Therapy 2002; 9: 804–813.
Lai CM, Spilsbury K, Brankov M, Zaknich T, Rakoczy PE . Inhibition of corneal neovascularization by recombinant adenovirus mediated antisense VEGF RNA. Exp Eye Res 2002; 75: 625–634.
Campochiaro PA . Gene therapy for ocular neovascularization. Curr Gene Ther 2007; 7: 25–33.
Klausner EA, Peer D, Chapman RL, Multack RF, Andurkar SV . Corneal gene therapy. J Control Release 2007; 124: 107–133.
Vázquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M et al. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem 1999; 274: 23349–23357.
Ambati BK, Joussen AM, Ambati J, Moromizato Y, Guha C, Javaherian K et al. Angiostatin inhibits and regresses corneal neovascularization. Arch Ophthalmol 2002; 120: 1063–1068.
Wu PC, Liu CC, Chen CH, Kou HK, Shen SC, Lu CY et al. Inhibition of experimental angiogenesis of cornea by somatostatin. Graefes Arch Clin Exp Ophthalmol 2003; 241: 63–69.
Ma DH, Zhang F, Shi W, Yao JY, Hsiao CH, Wu HC et al. Expression of tissue inhibitor of metalloproteinase-4 in normal human corneal cells and experimental corneal neovascularization. Ophthalmic Res 2003; 35: 199–207.
Shafiee A, Penn JS, Krutzsch HC, Inman JK, Roberts DD, Blake DA . Inhibition of retinal angiogenesis by peptides derived from thrombospondin-1. Invest Ophthalmol Vis Sci 2000; 41: 2378–2388.
Shao C, Sima J, Zhang SX, Jin J, Reinach P, Wang Z et al. Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci 2004; 45: 1758–1762.
Dueñas Z, Torner L, Corbacho AM, Ochoa A, Gutiérrez-Ospina G, López-Barrera F et al. Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest Ophthalmol Vis Sci 1999; 40: 2498–2505.
Yoon KC, Ahn KY, Lee JH, Chun BJ, Park SW, Seo MS et al. Lipid-mediated delivery of brain-specific angiogenesis inhibitor 1 gene reduces corneal neovascularization in an in vivo rabbit model. Gene Therapy 2005; 12: 617–624.
Rosmarin AG, Resendes KK, Yang Z, Mc Millan JN, Fleming SL . GA-binding protein transcription factor: a review of GABP as an integrator of intracellular signaling and protein–protein interactions. Blood Cells Mol Dis 2004; 32: 143–154.
Batchelor AH, Piper DE, de la Brousse FC, Mcknight SL, Wolberger C . The structure of GABPalpha/beta: an ETS domain-ankyrin repeat heterodimer bound to DNA. Science 1998; 279: 1037–1041.
Hauck L, Kaba RG, Lipp M, Dietz R, von Harsdorf R . Regulation of E2F1-dependent gene transcription and apoptosis by the ETS-related transcription factor GABPγ1. Mol Cell Biol 2002; 22: 2147–2158.
O'Leary DA, Koleski D, Kola I, Hertzog PJ, Ristevski S . Identification and expression analysis of alternative transcripts of the mouse GA-binding protein (Gabp) subunits alpha and beta1. Gene 2005; 344: 79–92.
Jeong BC, Kim MY, Lee JH, Kee HJ, Kho DH, Han KE et al. Brain-specific angiogenesis inhibitor 2 regulates VEGF through GABP that acts as a transcriptional repressor. FEBS Lett 2006; 580: 669–676.
Yang ZF, Mott S, Rosmarin AG . The Ets transcription factor GABP is required for cell-cycle progression. Nat Cell Biol 2007; 9: 339–346.
Okada Y, Yano K, Jin E, Funahashi N, Kitayama M, Doi T et al. A three-kilobase fragment of the human Robo4 promoter directs cell type-specific expression in endothelium. Circ Res 2007; 100: 1712–1722.
Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V et al. Roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci USA 2005; 102: 6373–6378.
Suchting S, Heal P, Tahtis K, Stewart LM, Bicknell R . Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB J 2005; 19: 121–123.
Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nature Med 2008; 14: 448–453.
Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ . A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci 1996; 37: 1625–1632.
Demir T, Celiker UO, Kükner A, Mogulkoç R, Celebi S, Celiker H . Effect of Octreotide on experimental corneal neovascularization. Acta Ophthalmol Scand 1999; 77: 386–390.
Knop E, Knop N . Anatomy and immunology of the ocular surface. Chem Immunol Allergy 2007; 92: 36–49.
Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006; 443: 993–997.
Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol 2004; 165: 2177–2185.
Hurmeric V, Mumcuoglu T, Erdurman C, Kurt B, Dagli O, Durukan AH . Effect of subconjunctival bevacizumab (Avastin) on experimental corneal neovascularization in guinea pigs. Cornea 2008; 27: 357–362.
Kim TI, Kim SW, Kim S, Kim T, Kim EK . Inhibition of experimental corneal neovascularization by using subconjunctival injection of bevacizumab (Avastin). Cornea 2008; 27: 349–352.
You IC, Kang IS, Lee SH, Yoon KC . Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal noevascularization. Acta Ophthalmol 2009; 87 (in press).
Okada Y, Jin E, Nikolova-Krstevski V, Yano K, Liu J, Beeler D et al. A GABP-binding element in the Robo4 promoter is necessary for endothelial expression in vivo. Blood 2008; 112: 2336–2339.
Acknowledgements
We thank Hong-Jae Chae (Chonnam National University Hospital) for assisting with the statistical analysis. This work was supported by the Korea Science and Engineering Foundation through the Medical Research Center for Gene Regulation (R13-2002-013-04001-0) at Chonnam National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, K., Bae, J., Park, H. et al. Subconjunctival gene delivery of the transcription factor GA-binding protein delays corneal neovascularization in a mouse model. Gene Ther 16, 973–981 (2009). https://doi.org/10.1038/gt.2009.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.50
Keywords
This article is cited by
-
Therapeutic Strategies for Corneal Wound Angiogenesis
Current Pathobiology Reports (2020)
-
Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye
Pharmaceutical Research (2019)