Abstract
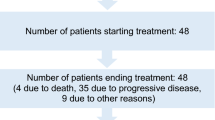
A phase-I trial to assess the safety and tolerability of human interleukin-12 (IL-12) plasmid (phIL-12) formulated with a synthetic lipopolymer, polyethyleneglycol–polyethyleneimine–cholesterol (PPC), was conducted on women with chemotherapy-resistant recurrent ovarian cancer. A total of 13 patients were enrolled in four dose-escalating cohorts and treated with 0.6, 3, 12 or 24 mg m−2 of the formulated plasmid once every week for 4 weeks. Administration of phIL-12/PPC was generally safe and well-tolerated. Common side effects included low-grade fever and abdominal pain. Stable disease and reduction in serum CA-125 levels were clinically observed in some patients. Measurable levels of IL-12 plasmid were detectable in PF samples collected throughout the course of phIL-12/PPC treatment. In comparison, serum samples either did not contain detectable amounts of plasmid DNA or contained <1% of the amount found in the corresponding PF samples. Treatment-related increases in IFN-γ levels were observed in PF but not in serum. These data demonstrate that IL-12 gene delivery with a synthetic delivery system is feasible for ovarian cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
American Cancer Society (2007). Cancer Facts and Figures 2007. American Cancer Society: Atlanta, 2007.
Chobanian N, Dietrich CS . Ovarian cancer. Surg Clin North Am 2008; 88: 285–299.
Bhoola S, Alvarez R . Novel therapies for recurrent ovarian cancer management. Expert Rev Anticancer Ther 2004; 4: 437–448.
Oei L, Sweep FC, Thomas CM, Boerman OC, Massuger LF . The use of monoclonal antibodies for the treatment of epithelial ovarian cancer. Int J Oncol 2008; 32: 1145–1157.
Chu CS, Kim SH, June CH, Coukos G . Immunotherapy opportunities in ovarian cancer. Expert Rev Anticancer Ther 2008; 8: 243–257.
Hogberg T, Glimelius B, Nygree P . A systematic overview of chemotherapy effects in ovarian cancer. Acta Oncol 2001; 40: 340–360.
Robertson MJ, Ritz J . Interleukin-12: basic biology and potential applications in cancer treatment. Oncologist 1996; 1: 88–97.
Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing The cells. J Exp Med 1993; 177: 1199–1204.
Colombo MP, Trinchieri G . Interleukin-12 in antitumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13: 155–168.
Trinchieri G, Gerosa F . Immunoregulation by interleukin-12. J Leuk Biol 1996; 59: 505–511.
Manetti R, Gerosa F, Giudizi MG, Biagiotti R, Parronchi P, Piccinni MP et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med 1994; 179: 1273–1283.
Wigginton JM, Gruys E, Geiselhart L, Subleski K, Komschlies KL, Park JW et al. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest 2001; 108: 51–62.
Murphy WJ, Welniak L, Back T, Hixon J, Subleski J, Seki N et al. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J Immunol 2003; 170: 2727–2733.
Wigginton JM, Lee JK, Wiltrout TA, Alvord WG, Hixon JA, Subleski J et al. Synergistic engagement of an ineffective endogenous anti-tumor immune response and induction of IFN-gamma and Fas-ligand-dependent tumor eradication by combined administration of IL-18 and IL-2. J Immunol 2002; 169: 4467–4474.
Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL . The role of Cxc chemokines as regulators of angiogenesis. Shock 1995; 4: 155–160.
Sgadari C, Angiolillo A, Tosato G . Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 1996; 87: 3877–3882.
Yu WG, Yamamoto N, Takenaka H, Mu J, Tai XG, Zou JP et al. Molecular mechanisms underlying IFN-g mediated tumor growth inhibition induced during tumor immunotherapy with rIL-12. Int Immunol 1996; 8: 855–865.
Guckek B, Meyer GC, Rudy W, Batrla R, Meuer SC, Bastert G et al. Interleukin-12 requires initial CD80-mediated T-cell activation to support immune responses toward human breast and ovarian carcinoma. Cancer Gene Ther 1999; 6: 228–237.
Ogawa M, Yu WG, Umehara K, Iwasaki M, Wijesuriya R, Tsujimura T et al. Multiple role of interferon-gamma in the mediation of interleukin 12-induced tumor regression. Cancer Res 1998; 58: 2426–2432.
Silver DF, Hempling RE, Piver MS, Repasky EA . Effects of IL-12 on human ovarian tumors engrafted into SCID mice. Gynecol Oncol 1999; 72: 154–160.
DeCesare SL, Michelini-Norris B, Blanchard DK, Barton DP, Cavanagh D, Roberts WS et al. Interleukin-12-mediated tumoricidal activity of patient lymphocytes in an autologous in vitro ovarian cancer assay system. Gynecol Oncol 1995; 57: 86–95.
Sanches R, Kuiper M, Penault-Llorca F, Aunoble B, D’Incan C, Bignon YJ . Antitumoral effect of interleukin-12-secreting fibroblasts in a mouse model of ovarian cancer: implications for the use of ovarian cancer biopsy-derived fibroblasts as a vehicle for regional gene therapy. Cancer Gene Ther 2000; 7: 707–720.
Akira M, Yamasaki S, Inoue N, Yang W, Nakau M, Yasuda S . Interleukin-12-gene transduction makes DCs from tumor-bearing mice an effective inducer of tumor-specific immunity in a peritoneal dissemination model. Immunol Lett 2002; 83: 13–20.
Lenzi R, Rosenblum M, Verschraegen C, Kudelka AP, Kavanagh JJ, Hicks ME et al. Phase I study of intraperitoneal recombinant human interleukin-12 in patients with Mullerian carcinoma, gastrointestinal primary malignancies, and mesothelioma. Clin Cancer Res 2002; 8: 3686–3695.
Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBio JS et al. Phase I trial evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res 1997; 3: 409–417.
Gollob JA, Mier JW, Veenstra K, McDermott DF, Clancy D, Clancy M et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-γ induction is associated with clinical response. Clin Cancer Res 2000; 6: 1678–1692.
Cebon J, Jager E, Shackleton MJ, Gibbs P, Davis ID, Hopkins W et al. Two phase I studies of low dose recombinant human IL-12 with melan-A and influenza peptides in patients with advanced malignant melanoma. Cancer Immunol 2003; 16: 3–7.
Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, Sherman ML et al. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood 1999; 94: 902–908.
Bajetta E, Del Vecchio M, Mortarini R, Nadeau R, Rakhit A, Rimassa L et al. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin Cancer Res 1998; 4: 75–85.
Marshall E . Cancer trial of interleukin-12 halted. Science 1995; 268: 1555.
Fewell J, Matar M, Slobodkin G, Rice J, Hovanes B, Anwer K . Synthesis and application of biocompatible non-viral gene delivery system for immunogene therapy of cancer. J Control Release 2005; 109: 288–298.
Fewell JG, Rice JK, Matar M, Slobodkin G, Lewis DH, Anwer K . Combination interleukin-12 intraperitoneal gene therapy with chemotherapy for treatment of disseminated ovarian cancer. Proc Am Assoc Cancer Res 2006; 47: 2112.
Brunhoeber E, Matar M, Anwer K, Fewell J . Biodistribution and clearance following intraperitoneal injection of murine interleukin-12 plasmid formulated with a novel polymeric delivery system. Mol Ther 2006; 13 (Suppl 109): 286.
Fewell JG, Rice J, Matar M, Anwer K . Safety and toxicity following intraperitoneal injection of murine interleukin-12 plasmid formulated with a novel polymeric delivery system. Mol Ther 2006; 13 (Suppl 109): 287.
Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC . Catheter type, placement and insertion techniques for preventing peritonitis in peritoneal dialysis patients. Cochrane Database Syst Rev 2004; 4: CD004680.
Hortobagyi GN, Ueno NT, Xia W, Zhang S, Wolf JK, Putnam JB et al. Cationic liposome-mediated E1A gene transfer to human breast and ovarian cancer cells and its biological effects: a phase I clinical trial. J Clin Oncol 2001; 19: 4183–4184.
Fewell JG, Rice J, Matar M, Brunhoeber E, Pence C, Slobodkin G et al. Use of interleukin-12 gene nanocomplexes as a general treatment against disseminated peritoneal malignancies. Proc Am Assoc Cancer Res 2007; 48: 3304.
Fewell JG, Matar M, Rice JS, Brunhoeber E, Slobodkin G, Pence C et al. Treatment of disseminated ovarian cancer using nonviral interleukin-12 gene therapy delivered intraperitoneally. J Gene Med 2009; 11: 718–728.
Markman M . Intraperitoneal chemotherapy as primary treatment of advanced ovarian cancer: efficacy, toxicity, and future directions. Rev Recent Clin Trials 2007; 2: 169–173.
Armstrong DK, Bundy B, Wanzel L, Huang HQ, Baergen R, Shashikant L . Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34–43.
Lenzi R, Edward R, June C, Seiden MV, Garcia ME, Rosenblum M et al. Phase II study of intraperitoneal recombinant interleukin-12 (rhIL-12) in patients with peritoneal carcinomatosis (residual disease <1 cm) associated with ovarian cancer or primary peritoneal carcinoma. J Transl Med 2007; 5: 66–72.
Heinzerling L, Burg G, Dummer R, Maier T, Oberholzer PA, Schultz J et al. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum Gene Ther 2005; 16: 35–48.
Triozzi PL, Strong TV, Bucy RP, Allen KO, Carlisle RR, Moore SE et al. Intratumoral administration of a recombinant Canarypox virus expressing interleukin 12 in patients with metastatic melanoma. Hum Gene Ther 2005; 16: 91–100.
Sonabend AM, Velicu S, Ulasov IV, Han Y, Tyler B, Brem H et al. A safety and efficacy study of local delivery of IL-12 transgene by PPC polymer in a model of experimental glioma. Anticancer Drug 2008; 19: 133–142.
Orsi F, Vigna PD, Bonomo G, Belloni M . Percutaneous placement of peritoneal port-catheter in oncologic patients. Eur Radiol 2004; 14: 2020–2024.
Mosteller RD . Simplified calculation of body-surface area. N Engl J Med 1987; 317: 1098–1101.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216.
Acknowledgements
We thank Jolane Gable, Thelma Webb and Aparna Tamhane of the University of Alabama in Birmingham, Dr Marshall Schreeder of Clearview Cancer Center, Huntsville, Alabama, Dr Mathew Anderson of Baylor College of Medicine and Dirk Kieback for valuable contribution and input into this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anwer, K., Barnes, M., Fewell, J. et al. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther 17, 360–369 (2010). https://doi.org/10.1038/gt.2009.159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.159
Keywords
This article is cited by
-
Interleukin-12 as an in situ cancer vaccine component: a review
Cancer Immunology, Immunotherapy (2022)
-
Dual-targeted NIS polyplexes—a theranostic strategy toward tumors with heterogeneous receptor expression
Gene Therapy (2019)
-
History of Polymeric Gene Delivery Systems
Topics in Current Chemistry (2017)
-
Preclinical validation: LV/IL-12 transduction of patient leukemia cells for immunotherapy of AML
Molecular Therapy - Methods & Clinical Development (2016)