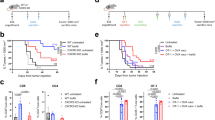
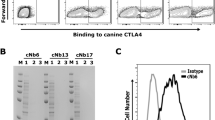
Abstract
Cell-based vaccination strategies to induce functional tumor-specific T cells in cancer patients have focused on using autologous dendritic cells. An alternative approach is to use RNA-loaded CD40 activated B cells (CD40-B) that are highly efficient antigen-presenting cells capable of priming naive T cells, boosting memory T-cell responses and breaking tolerance to tumor antigens. The use of tumor RNA as the antigenic payload allows for gene transfer without viruses or vectors and permits major histocompatibility complex (MHC)-independent, multiple-antigen targeting. Here, we use CD40L transfected K562 cells to generate functional CD40-B cells from the peripheral blood of humans and dogs. Testing of RNA-loaded CD40-B cells in dogs allows not only for its development in veterinary medicine but also for determination of its safety and efficacy in a large animal model of spontaneous cancer prior to initiation of human clinical trials. We found that CD40-B cells from healthy humans, healthy dogs and tumor-bearing dogs express increased levels of immune molecules such as MHC and CCR7. Moreover, RNA-loaded CD40-B cells induce functional, antigen-specific T cells from healthy dogs and dogs with lymphoma. These findings pave the way for immunotherapy trials using tumor RNA-loaded CD40-B cells to stimulate antitumor immunity in a large animal model of spontaneous neoplasia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Steinman RM, Dhodapkar M . Active immunization against cancer with dendritic cells: the near future. Int J Cancer 2001; 94: 459–473.
Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood 2002; 99: 1517–1526.
Brossart P, Wirths S, Brugger W, Kanz L . Dendritic cells in cancer vaccines. Exp Hematol 2001; 29: 1247–1255.
Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996; 2: 52–58.
Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4: 328–332.
Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 1999; 190: 1669–1678.
Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 2001; 61: 6451–6458.
Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA 2001; 98: 8809–8814.
Steinman RM . Dendritic cells and immune-based therapies. Exp Hematol 1996; 24: 859–862.
Jefford M, Maraskovsky E, Cebon J, Davis ID . The use of dendritic cells in cancer therapy. Lancet Oncol 2001; 2: 343–353.
Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 2002; 109: 409–417.
Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J et al. CD40 activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest 1997; 100: 2757–2765.
Coughlin CM, Vance BA, Grupp SA, Vonderheide RH . RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood 2004; 103: 2046–2054.
Rousset F, Garcia E, Banchereau J . Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med 1991; 173: 705–710.
Schultze JL, Seamon MJ, Michalak S, Gribben JG, Nadler LM . Autologous tumor infiltrating T cells cytotoxic for follicular lymphoma cells can be expanded in vitro. Blood 1997; 89: 3806–3816.
Ritchie DS, Yang J, Hermans IF, Ronchese F . B-Lymphocytes activated by CD40 ligand induce an antigen-specific anti-tumour immune response by direct and indirect activation of CD8(+) T-cells. Scand J Immunol 2004; 60: 543–551.
Coughlin CM, Fleming MD, Carroll RG, Pawel BR, Hogarty MD, Shan X et al. Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J Clin Oncol 2006; 24: 5725–5734.
Ting-De Ravin SS, Kennedy DR, Naumann N, Kennedy JS, Choi U, Hartnett BJ et al. Correction of canine X-linked severe combined immunodeficiency by in vivo retroviral gene therapy. Blood 2006; 107: 3091–3097.
Bauer Jr TR, Hai M, Tuschong LM, Burkholder TH, Gu YC, Sokolic RA et al. Correction of the disease phenotype in canine leukocyte adhesion deficiency using ex vivo hematopoietic stem cell gene therapy. Blood 2006; 108: 3313–3320.
Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther 2005; 12: 1072–1082.
Ponder KP, Melniczek JR, Xu L, Weil MA, O'Malley TM, O'Donnell PA et al. Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci USA 2002; 99: 13102–13107.
Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med 1999; 5: 56–63.
Thomas ED, Plain GL, Graham TC, Ferrebee JW . Long-term survival of lethally irradiated dogs given homografts of bone marrow. Blood 1964; 23: 488–493.
Mannick JA, Lochte Jr HL, Ashley CA, Thomas ED, Ferrebee JW . Autografts of bone marrow in dogs after lethal total-body radiation. Blood 1960; 15: 255–266.
Ferrebee JW, Lochte Jr HL, Jaretzki 3rd A, Sahler OD, Thomas ED . Successful marrow homograft in the dog after radiation. Surgery 1958; 43: 516–520.
Teske E . Canine malignant lymphoma: a review and comparison with human non-Hodgkin's lymphoma. Vet Q 1994; 16: 209–219.
Owen LN, Bostock DE, Halliwell RE . Cell-mediated and humoral immunity in dogs with spontaneous lymphosarcoma. Eur J Cancer 1975; 11: 187–191.
MacEwen EG . Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev 1990; 9: 125–136.
Bednarek MA, Sauma SY, Gammon MC, Porter G, Tamhankar S, Williamson AR et al. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. J Immunol 1991; 147: 4047–4053.
Hirama K, Togashi K, Wakasa C, Yoneda M, Nishi T, Endo Y et al. Cytotoxic T-lymphocyte activity specific for hemagglutinin (H) protein of canine distemper virus in dogs. J Vet Med Sci 2003; 65: 109–112.
Henry CJ, McCaw DL, Brock KV, Stoker AM, Tyler JW, Tate DJ et al. Association between cancer chemotherapy and canine distemper virus, canine parvovirus, and rabies virus antibody titers in tumor-bearing dogs. J Am Vet Med Assoc 2001; 219: 1238–1241.
Sixt N, Cardoso A, Vallier A, Fayolle J, Buckland R, Wild TF . Canine distemper virus DNA vaccination induces humoral and cellular immunity and protects against a lethal intracerebral challenge. J Virol 1998; 72: 8472–8476.
Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33.
Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM . The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol 1999; 162: 3859–3864.
von Bergwelt-Baildon M, Shimabukuro-Vornhagen A, Popov A, Klein-Gonzalez N, Fiore F, Debey S et al. CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood 2006; 107: 2786–2789.
Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005; 438: 803–819.
Gantner F, Hermann P, Nakashima K, Matsukawa S, Sakai K, Bacon KB . CD40-dependent and -independent activation of human tonsil B cells by CpG oligodeoxynucleotides. Eur J Immunol 2003; 33: 1576–1585.
Ponce F, Magnol JP, Ledieu D, Marchal T, Turinelli V, Chalvet-Monfray K et al. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet J 2004; 167: 158–166.
Salter RD, Howell DN, Cresswell P . Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics 1985; 21: 235–246.
Vonderheide RH, Hahn WC, Schultze JL, Nadler LM . The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity 1999; 10: 673–679.
Vonderheide RH, Schultze JL, Anderson KS, Maecker B, Butler MO, Xia Z et al. Equivalent induction of telomerase-specific cytotoxic T lymphocytes from tumor-bearing patients and healthy individuals. Cancer Res 2001; 61: 8366–8370.
Zuker M . Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31: 3406–3415.
Spichiger AC, Allenspach K, Ontsouka E, Gaschen F, Morel C, Blum JW et al. Abundance of mRNA of growth hormone receptor and insulin-like growth factors-1 and -2 in duodenal and colonic biopsies of dogs with chronic enteropathies. J Vet Med A Physiol Pathol Clin Med. 2005; 52: 491–497.
Acknowledgements
We thank Ms Amy LaBlanc, Ms Rachel Cohen, Ms Roxanne Buchanan and Ms Patty O'Donnell for their excellent technical assistance and Dr William Lee and Dr Carl June for helpful discussions and mentorship. This work was supported by grants from the Alliance for Cancer Gene Therapy (to RHV), the Barry and Savannah Poodle Memorial Fund (to NJM) and institutional funds from the University of Pennsylvania Abramson Cancer Center (to RHV) and the University of Pennsylvania School of Veterinary Medicine (to NJM and KUS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mason, N., Coughlin, C., Overley, B. et al. RNA-loaded CD40-activated B cells stimulate antigen-specific T-cell responses in dogs with spontaneous lymphoma. Gene Ther 15, 955–965 (2008). https://doi.org/10.1038/gt.2008.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.22
Keywords
This article is cited by
-
The immunosuppressive factors IL-10, TGF-β, and VEGF do not affect the antigen-presenting function of CD40-activated B cells
Journal of Experimental & Clinical Cancer Research (2012)