Abstract
Dendritic cell (DC)-based immunotherapy has been investigated as a new therapeutic approach to intractable neuroblastomas; however, only limited clinical effect has been reported. To overcome the relatively low sensitivity of neuroblastomas against immunotherapy, we undertook a preclinical efficacy study to examine murine models to assess the combined effects of γ-irradiation pretreatment and recombinant Sendai virus (ts-rSeV/dF)-mediated murine interferon-β (mIFN-β) gene transfer to DCs using established c1300 neuroblastomas. Similar to intractable neuroblastomas in the clinic, established c1300 tumors were highly resistant to monotherapy with either γ-irradiation or DCs activated by ts-rSeV/dF without transgene (ts-rSeV/dF-null) that has been shown to be effective against other murine tumors, including B16F10 melanoma. In contrast, immunotherapy using DCs expressing mIFN-β through ts-rSeV/dF (ts-rSeV/dF-mIFNβ-DCs) effectively reduced tumor size, and its combination with γ-irradiation pretreatment dramatically enhanced its antitumor effect, resulting frequently in the complete elimination of established c1300 tumors 7–9 mm in diameter, in a high survival rate among mice, and in the development of protective immunity in the mice against rechallenge by the tumor cells. These results indicate that the combination of ts-rSeV/dF-mIFNβ-DCs with γ-irradiation is a hopeful strategy for the treatment of intractable neuroblastomas, warranting further investigation in the clinical setting.
Similar content being viewed by others
Introduction
Neuroblastoma, with its many clinical and molecular faces, is the most common extracranial malignant solid tumor seen in children.1 When occurring in infants less than 1 year old, neuroblastoma shows a relatively good prognosis, whereas only 30% of children above that age with advanced cases of the disease do not experience disease progression for at least 3 years after treatment.2 Recent efforts by physicians have demonstrated that surgical intervention, irradiation and intensive chemotherapy followed by stem cell transplantation improved the survival of such patients.3, 4 However, in a large number of these children, especially in cases with MYCN amplification, the disease remains intractable.3, 4
As an alternative potential therapy, clinical evaluation of dendritic cell (DC)-based immunotherapy was initiated several years ago. The first reported clinical study, which enrolled 15 children with advanced solid tumors including three individuals with neuroblastoma, demonstrated modest antitumor responses.5 Subsequently, another group reported the results of tumor RNA-loaded DC vaccination for 11 patients with stage 4 neuroblastoma.6 Even though these challenging clinical studies showed specific antitumor immune reactions, the clinical outcome is still far from the level required for a standard therapy.
Very importantly, these frontier studies suggested that the immunosuppressed condition of these patients after intensive chemotherapy might limit the efficacy of DC-based immunotherapy.6 In addition, neuroblastoma is shown to be less immunogenic,7 in association with a suppressed expression of major histocompatibility complex class I, which can be caused by MYCN amplification.8, 9, 10 DC-based cancer immunotherapy, moreover, is now also a developing technology and has shown limited clinical outcome in other malignancies. Therefore, scientists and physicians should elucidate (1) the most effective DC subtypes, (2) the optimal conditions and activation stimuli to generate activated DCs showing optimal antitumor effects in vivo, (3) the optimal route for administration and (4) the optimal dose and frequency of DC vaccinations.11, 12, 13 The lack of such information may explain the limited efficacy of DC-based immunotherapy for advanced neuroblastoma in the clinical setting.
Recently, we have demonstrated a dramatic improvement in the efficacies of DCs activated by recombinant Sendai virus (rSeV), namely ‘immunostimulatory virotherapy’, on multiple syngeneic mouse models bearing highly malignant tumors, including B16F10 melanoma,14 MH134 hepatocellular carcinoma14 and SCCVII squamous cell carcinoma.15 rSeV is a novel and powerful gene transfer modality as a cytoplasmic gene expression system16, 17, 18 that leads DCs to highly activated/mature state through a DExD/H-box RNA helicase, retinoic acid-inducible gene-I (RIG-I).19, 20 Therefore, we hypothesized that rSeV-activated DCs might enhance antitumor immunity against less immunogenic c1300 neuroblastoma.
With this background in mind, we here examined and optimized the antitumor effect of DC immunotherapy activated by a ‘temperature-sensitive mutant’ and F-gene-deleted non-transmissible rSeV (ts-rSeV/dF), an advanced vector design showing a less cytopathic effect,18, 21, 22 that is now available for mass production according to the good manufacturing practice guidelines.
Our goal was to develop therapeutics based on ts-rSeV/dF-DCs that could completely eliminate, rather than merely shrink, established tumors as well as induce protective antitumor immunity against recurrence. Importantly, although here we confirmed that the c1300 tumor was still highly resistant to DC-based immunotherapy, even with the use of DCs activated by ts-rSeV/dF, we here found that γ-irradiation pretreatment accompanying murine interferon-β (mIFN-β) gene transfer dramatically and synergistically enhanced the antitumor immunity induced by intratumoral (i.t.) injection of ts-rSeV/dF-DCs without any antigen loading ex vivo. We here show that this new regimen resulted not only in the complete elimination of a high proportion of established c1300 tumors 7–9 mm in diameter, but also in the induction of tumor-specific protective immunity.
Results
c1300 neuroblastoma is highly resistant to DC-based immunotherapy
Throughout this study, DCs were not pulsed by tumor antigen ex vivo, because antigen loading did not enhance the antitumor effect of ts-rSeV/dF-DCs that were administered i.t. to c1300 tumors (data not shown). These findings were similar to those of our earlier studies using B16 melanoma14 and SCCVII squamous cell carcinoma.15
To optimize the dose of DC-based immunotherapy that was activated by ts-rSeV/dF-null (ts-rSeV/dF-DCs), we first determined the effective dose of DCs to dermally implanted c1300 neuroblastomas in the abdominal wall of A/J female mice. We here administered ts-rSeV/dF-DCs without antigen pulsation i.t., because this injection route showed an optimal antitumor effect against both B16F10 melanoma14 and SCCIIV squamous cell carcinoma15 in our earlier studies. At the same time, the therapeutic effect against c1300 was directly compared with that against B16F10 melanoma.
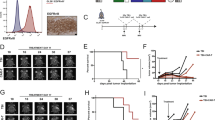
As shown in Figure 1, in the ‘early treatment regimen’,14 three-times weekly administration of ts-rSeV/dF-DCs, the highest efficacy against B16F10 melanoma occurred at 106 cells per dose of ts-rSeV/dF-DCs, resulting in the elimination of 50% of the tumors. In contrast, ts-rSeV/dF-DCs showed a modest suppression of tumor growth of c1300 neuroblastomas without eliminating any of the tumors, suggesting that ts-rSeV/dF-DC-based immunotherapy was more effective against B16F10 than against c1300.
Direct comparison of antitumor effect by direct and repeated i.t. injection of DCs activated by temperature-sensitive mutant and F-gene-deleted non-transmissible recombinant Sendai virus (ts-rSeV/dF-DCs) without any therapeutic gene. Three days after intradermal inoculation of B16F10 melanoma (left panels) or c1300 neuroblastoma (right panels), various amounts of ts-rSeV/dF-DCs were injected weekly through an i.t. route as the ‘early’ treatment regimen. Thereafter, the tumor volume was measured. Lines on the panels indicate time courses of tumor volume in individual animals. Apparent dose-efficacy response was seen in the tumor volume of B16F10 melanoma, and four of eight animals that received 106 DCs per dose showed complete tumor elimination (left panels). In contrast, no animals inoculated with c1300 neuroblastoma demonstrated either complete elimination or significant dose–response on tumor size at any dose. The + symbol indicates animals that died during observation. DC, dendritic cell; i.t., intratumoral; rSeV, recombinant Sendai virus.
Gene transfer of mIFN-β by ts-rSeV/dF to DCs enhances the antitumor effect against c1300
To overcome the limited efficacy of ts-rSeV/dF-DCs against c1300, we next examined the effect of mIFN-β gene transfer by ts-rSeV/dF to DCs (ts-rSeV/dF-mIFNβ-DCs), which has been shown to enhance antitumor immunity to B16 melanoma effectively.14
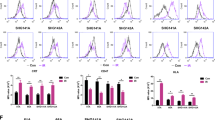
As shown in Figure 2a, recombinant mIFN-β or human IFN-β was effective in upregulating major histocompatibility complex class I antigen expression not only in murine (c1300) but also in human (SK-N-SH and IMR32) neuroblastoma cell lines, irrespective of predefined N-myc amplification. However, direct cytoxicity had no or only a modest effect on them (Figure 2b). These findings were similar to those of our earlier study obtained by the use of melanomas14 and indicated the modest susceptibility of c1300 neuroblastomas to mIFN-β.
Interferon-β strongly upregulates MHC class I molecules of, and shows no or modest cytotoxicity against, murine and human neuroblastomas. c1300 (mouse), SK-N-SH (human) or IMR32 (human) neuroblastoma was treated with IFN-β at 1000 U ml−1 for corresponding species (c1300 for mIFN-β and SK-N-SH or IMR32 for hIFN-β, respectively) for 48 h. (a) Expression of MHC class I molecule. The panels are the typical FACS patterns among three independent experiments. C1300 cells abundantly expressed MHC class I, and mIFN-β upregulated mean fluorescence intensity (MFI). SK-N-SH cells show no expression of MHC class I, and hIFN-β stimulated its expression. IMR32 indicated dual peaks of MHC class I expression, suggesting that this cell line was composed of heterogeneous populations, and hIFN-β treatment strongly induced MHC class I expression. (b) In vitro cytotoxicity assay for IFN-βs to murine neuroblastoma (yellow line) and human neuroblastomas (SK-N-SH: blue line and IMR32: red line). The viability of SK-N-SH cells was not affected by hIFN-β, and c1300 and IMR32 showed modest (∼20%) cytotoxicity by IFN-β treatment. hIFN-β, human IFN-β; MHC, major histocompatibility complex; mIFN-β, murine interferon-β. (See online version for color figure.)
In turn, the use of ts-rSeV/dF-mIFNβ, instead of the ts-rSeV/dF-null vector, as a DC activator dramatically enhanced the antitumor effect on c1300, resulting in the elimination of 60% of the tumors in the ‘early’ treatment regimen in vivo, as expected (Figure 3a, left three panels and Figure 3b, left graph).
Enhanced antitumor activity against established c1300 neuroblastoma in vivo by ts-rSeV/dF-DCs expressing mIFN-β. Three days (‘early’ treatment regimen) or 10 days (‘later’ treatment regimen) after intradermal inoculation of c1300 neuroblastomas, 106 cells of ts-rSeV/dF-DCs with or without exogenous mIFN-β expression were injected weekly through the i.t. route. Thereafter, the tumor volume was measured. Note that all tumors treated in the ‘later’ regimen were over 7 mm in diameter at day 10. (a) Time courses of tumor volume in individual animals by ‘early’ (left panels) and ‘later’ (right panels) treatment regimens. The c1300 tumors were still resistant to the weekly treatment with ts-rSeV/dF-DCs in both regimens (middle two panels), and apparent efficacies with regard to volume reduction and tumor elimination were found in the use of ts-rSeV/dF-DC-associated exogenous mIFN-β. (b) Panels showing c1300 tumor volume on day 31 in the animals demonstrated in (a). Note that animals showing a complete elimination of tumors were excluded from these analyses. Treatment with ts-rSeV/dF-DCs significantly inhibited tumor growth, and the expression of exogenous mIFN-β strongly reduced the tumor size in both regimens. *P<0.001. DC, dendritic cell; mIFN-β, murine interferon-β; rSeV, recombinant Sendai virus.
We then asked whether or not established c1300 tumors, 7–9 mm in diameter, could respond to ts-rSeV/dF-mIFNβ-DCs through the ‘later’ treatment regimen starting at 10 days after tumor inoculation. Although ts-rSeV/dF-mIFNβ-DCs significantly suppressed the growth of established c1300 tumors, the percentage of tumors eliminated was still and unexpectedly low (one of six animals showed complete elimination; Figure 3a, right three panels and Figure 3b, right graph). In this experiment, no animal except the one showing tumor elimination survived over 120 days (data not shown; the representative data are shown in Figure 5b), indicating that rSeV/dF-mIFNβ-DCs contributed to the significant suppression of tumor growth, though not enough to prolong the survival of tumor-bearing mice.
Combination therapy of irradiation and rSeV/dF-mIFNβ-DC induces long-lasting and tumor-specific protective immunity. (a) Assessment of CTL activity for c1300. Induction of tumor-specific CTLs after i.t. administration of rSeV/dF-mIFNβ-DC, which was repeated twice according to the late treatment regimen. Control included tumor-bearing mice without any treatment and MuSS was also used as a target of a third party. Seven days after the last treatment, splenocytes were isolated and restimulated in vitro for 5 days with mitomycin C-treated MH134 cells, and cytolytic activity against 51Cr-labeled targets was measured. Each group contains n=3. (b) Seven days after s.c. c1300 tumor inoculation into the left thigh, the tumors were irradiated three times daily at 4 Gy day−1. At day 10 (‘later’ treatment regimen), 106 cells of ts-rSeV/dF-DCs with exogenous mIFN-β expression were injected weekly through the i.t. route. Four animals with completely eliminated tumors through overdose irradiation (34 Gy × 3 for 3 days) were also included. On day 184, live animals without primary tumor formation were subjected to a second challenge, simultaneous tumor inoculation with c1300 and MuSS (third party) on the abdominal wall. Fifteen days later, tumor formation was determined. DC, dendritic cell, i.t., intratumoral; mIFN-β, murine interferon-β; rSeV, recombinant Sendai virus; s.c., subcutaneous injection. (See online version for color figure.)
Synergistic sensitization of c1300 tumor to ts-rSeV/dF-IFNβ-DCs by γ-irradiation pretreatment
Together, these data confirmed the limited efficacy of ts-rSeV/dF-DC-based immunotherapy against less-immunogenic c1300 neuroblastoma. We therefore next looked for a possible sensitizer that might enhance the effect of DC immunotherapy. Recent studies have demonstrated that radiotherapy induces an ‘abscopal effect’ against distant tumors,23, 24 probably due to the enhancement of antitumor immunity.25 We therefore examined the combined effect of a clinically available dose of γ-irradiation (4 Gy day−1 for 3 days) followed by weekly i.t. administration of rSeV/dF-mIFNβ-DCs in the ‘later’ treatment regimen (Figure 4, scheme). In this experiment, the tumor was intradermally implanted in the thigh to avoid radiation-induced toxicity to vital organs.
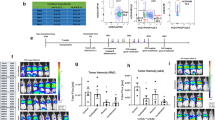
Pretreatment of clinically available dose of irradiation dramatically enhanced antitumor effect of ts-rSeV/dF-mIFNβ-DCs seen in the ‘later’ treatment regimen. Seven days after s.c. tumor inoculation into the left thigh, the tumors were irradiated three times daily at 4 Gy day−1. At day 10 (‘later’ treatment regimen), 106 cells of ts-rSeV/dF-DCs with exogenous mIFN-β expression were injected weekly through the i.t. route. Thereafter, the tumor volume was measured. Note that all tumors treated in the ‘later’ regimen were over 7 mm in diameter at day 10. (a) Time courses of tumor volume in individual animals. No tumor disappeared in the group receiving radiation only, but tumor growth was suppressed (right upper panel) compared with those without any treatment (left upper). In contrast, the ts-rSeV/dF-mIFNβ-DC group showed a strong suppression of tumor growth (representative findings seen in Figure 3), and this effect was dramatically enhanced by the combination therapy, which resulted in the elimination of a high percentage of tumors (6/8=75%). (b) Direct comparison of tumor size. Panels showing c1300 tumor volume on day 38 in the animals demonstrated in (a). Note that animals showing a complete elimination of tumors were excluded in these analyses. Treatment with either irradiation or ts-rSeV/dF-mIFNβ-DCs significantly inhibited tumor growth, and synergism was found by the combination therapy. *P<0.001 and #P<0.05. (c) Long-term survival of animals demonstrated in (a). Two animals in the rSeV/dF-mIFNβ-DC group that showed the complete disappearance of tumors survived over 200 days (2/8=25%), whereas no significant prolongation of survival was seen in the group treated with irradiation alone. The combination therapy dramatically and significantly improved the survival of animals; six of eight animals (75%) survived over 200 days without any recurrence. The data were analyzed by the Kaplan–Meier method, and statistical relevance was determined using the log-rank test. *P<0.001. DC, dendritic cell; i.t., intratumoral; mIFN-β, murine interferon-β; rSeV, recombinant Sendai virus; s.c., subcutaneous injection. (See online version for color figure.)
As shown in Figures 4a and b, monotherapy consisting of either γ-irradiation or rSeV/dF-mIFNβ-DCs effectively reduced the tumor volume. However, the established tumors were rarely eliminated (γ-irradiation: 0/6 animals; rSeV/dF-mIFNβ-DCs: 2/8 animals). In contrast, when these therapies were combined, six of eight animals showed the complete elimination of all established tumors at 38 days after inoculation. As the result, 5 of the 8 animals treated with both γ-irradiation and rSeV/dF-mIFNβ-DCs survived over 200 days in tumor-free condition (P<0.001 vs other groups) (Figure 4c).
Immunotherapy using ts-rSeV/dF-IFNβ-DCs was required to establish protective immunity for the second challenge of c1300 tumor cells
Finally, we asked whether or not the complete elimination of c1300 tumors might contribute to the establishment of long-lasting protective immunity.
At first, we assessed the cytotoxic T-lymphocyte (CTL) activity using splenocytes from mice with DC treatment. A strong and c1300-specific cell lytic activity of stimulated splenocytes with tumor antigen was found only in the case of mice with combined treatment, but not with other treatment groups (Figure 5a, left graph). Such a cell lytic activity could not be found when MuSS (a third-party tumor: A/J mouse-derived malignant fibrous histiocytoma)26 was used as the target (Figure 5a, right graph).
Next, we performed an additional experiment for the second challenge by the simultaneous inoculation of c1300 and MuSS on day 186. The overdose irradiation group (34 Gy × 3 days) that showed a high percentage of c1300 tumor elimination (>70%, according to our repeated preliminary study) was also included as a control group.
As shown in Figure 5b, none of the animals bearing established c1300 tumors without any treatment or with a clinically available dose of radiation (4 Gy × 3 days) survived over 120 days after tumor inoculation. Only one of four animals treated with rSeV/dF-mIFNβ-DCs showed tumor-free survival over 120 days, but this mouse accepted not only third-party MuSS but also c1300 at the second challenge. Three of the four tumor-bearing mice that were treated with overdose irradiation survived over 120 days in tumor-free condition, but no mouse could reject both MuSS and c1300, indicating that tumor elimination by γ-irradiation itself does not significantly contribute to the establishment of protective immunity. In contrast, combination therapy of radiation and rSeV/dF-mIFNβ-DCs also resulted in tumor elimination and tumor-free survival over 120 days in three of the four animals, and all the four mice demonstrated complete and tumor-specific rejection to c1300 tumor inoculation, but none of them showed such a rejection of third-party, MuSS cells.
These results indicate that the synergism of combining radiation and rSeV/dF-mIFNβ-DCs contributes greatly not only to tumor reduction but also to the establishment of long-lasting and protective antitumor immunity.
Discussion
Since the early reports describing the efficacy of DC-based immunotherapy on subjects with malignancies,27, 28 this therapeutic mode has been evaluated all over the world. Recurrent neuroblastoma, which is highly resistant to currently available surgery, chemotherapy and radiotherapy, has also been a target of DC-based immunotherapy; but recent clinical studies have failed to show significant improvements in outcome.5, 6 To overcome the current limitation of this mode, we recently developed a new concept, ‘immunostimulatory virotherapy’, using rSeVs, which are recombinant virus-based immune boosters for DCs.14, 15 DCs activated by an rSeV have shown apparently superior antitumor effects on several tumor types compared with those seen by DCs treated with conventional stimuli, including lipopolysaccharide; they have also been shown not to lose their phago/pinocytotic activity,15 and therefore an i.t. injection of rSeV-DCs without exposure to tumor antigen ex vivo evoked tumor-specific antitumor immunity.14, 15 On the basis of these findings, the present experimental study was performed to examine the potential of DC-based immunotherapy boosted by a newly developed rSeV/dF, the less cytotoxic, clinically available vector ts-rSeV/dF,18 to treat less-immunogenic murine c1300 neuroblastoma.
The key observations obtained in this study were as follows: (1) c1300 tumors were highly resistant to ts-rSeV/dF-DC therapy by the ‘early’ treatment regimen that was sufficiently effective against highly malignant B16F10 melanoma, indicating that c1300 should be less immunogenic than such melanoma; (2) the use of ts-rSeV/dF-mIFNβ as the activating modality for DCs and expressing murine IFN-β dramatically attenuated the antitumor effect on c1300 tumors through the ‘early’ treatment regimen, similar to a finding of our earlier study;14 (3) when established c1300 tumors were treated through the ‘later’ treatment regimen, however, the antitumor effect of ts-rSeV/dF-mIFNβ-DCs was not sufficient; (4) radiation pretreatment at a clinically reasonable dose (4 Gy × 3 days) demonstrated a dramatically improved antitumor effect, resulting in a high percentage of elimination of established tumors; and (5) the elimination of established c1300 tumors by radiation followed by ts-rSeV/dF-mIFNβ-DCs contributed to the development of long-lasting tumor-specific immunity, whereas the antitumor effect through overdose irradiation did not. These results indicate the potential utility of the combination of radiotherapy and ts-rSeV/dF-mIFNβ-DC immunogene therapy in the clinical setting.
It has been suggested that radiotherapy for malignancies might stimulate antitumor immunity as a systemic bystander effect called the ‘abscopal effect’. However, the molecular and cellular mechanisms underlying this effect are largely unknown. In addition, the concept of combining radiation therapy with immunotherapy is not new, and some publications suggest the beneficial effect of irradiation on antitumor immunity. Radiation induces cell death through apoptosis and necrosis. In turn, necrotic and apoptotic cells could induce DC-mediated antitumor immunity.29, 30, 31 These types of cell death are also shown not only to induce the release of inflammatory cytokines,32 but also to stimulate tumor vasculature to upregulate the expression of adhesion molecules, and to facilitate the trafficking of immune cells to cancer foci; thus, these types of cell death may together be responsible for the abscopal effect after radiation therapy.
It is of interest, however, that abscopal regression of distant tumors has been inferred in the use of certain tumors in experimental studies, but has rarely been seen in clinical settings.33 In fact, we here demonstrated that tumor elimination through overdose irradiation did not protect against the second challenge of c1300 inoculation; rather, the addition of DC immunotherapy was required to establish a protective immunity. These findings could be supported by an important report describing that the induction of the T-lymphocyte-mediated abscopal antitumor response was tumor type specific.25 Therefore, we concluded that DC-based immunotherapy would be required to induce protective immunity when tumor cells were destroyed by irradiation.
Related to this point, we have to discuss why c1300 tumor elimination through overdose irradiation (34 Gy × 3) did not induce protective immunity against the second challenge. In this case, a sustained dermal inflammation and burns were found (data not shown), possibly implying that the sustained dermal inflammation due to overdose irradiation might disturb the establishment of antitumor immunity. Therefore, clinically appropriate doses of irradiation, probably associated with tumor cell disruption proper for antigen uptake, processing and presentation by antigen-presenting cells, should be examined to obtain optimized antitumor immune responses following additional DC immunotherapy.
In summary, we here demonstrated that the i.t. injection of rSeV/DCs expressing mIFN-β to established c1300 tumors pretreated with a clinically reasonable dose of irradiation efficiently led tumors to a complete elimination in vivo. In addition, this regimen simultaneously induced a long-lasting protective immunity. Therefore, this results strongly suggest that the regimen warrants further investigation in research as well as in clinical trials.
Materials and methods
Mice and tumor cell lines
Female 6- to 8-week-old A/J mice (H-2a) were obtained from Japan SLC Inc. (Hamamatsu, Shizuoka, Japan) and kept under specific pathogen-free and humane conditions. Murine neuroblastoma c1300 and MuSS murine malignant fibrous histiocytoma (under kind permission by Dr Itaru Watanabe, Department of Surgery, Tasuda Hospital)26 were obtained from the RIKEN BioResearch Center (Tsukuba, Ibaraki, Japan). B16F10 murine melanoma, SK-N-SH human neuroblastoma without MYCN amplification and IMR32 human neuroblastoma associated with MYCN amplification were purchased from ATCC (Manassas, VA, USA). These cell lines were maintained in complete medium (RPMI-1640 medium; Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal calf serum (BioWest, Nuaille, France), penicillin and streptomycin under a humidified atmosphere containing 5% CO2 at 37 °C.
Temperature-sensitive mutant non-transmissible rSeVs (ts-rSeV/dF)
Temperature-sensitive mutant F-defective non-transmissible recombinant rSeVs (ts-rSeV/dF-null and ts-rSeV/dF-mIFNβ) were prepared and recovered as described earlier.21, 22 Briefly, vectors were prepared by using recombinant LLC-MK2 cells carrying the F gene (LLC-MK2/F7). An adenovirus vector, AxCANCre, expressing Cre recombinase was used for the induction of F protein in LLC-MK2/F7 cells (referred to as LLC-MK2/F7/A). Recombinant vaccinia virus vTF7-3 carrying T7 RNA polymerase was inactivated with psoralen and long-wave ultraviolet irradiation and then used for the ribonucleoprotein complex recovery. The viral vectors were further amplified by several rounds of propagation. The titers of the recovered viral vectors were expressed as cell infectious units.9 Murine IFN-β cDNA, which was subcloned to the vector template ts-prSeV18+b(+)/dF, was cloned by reverse transcriptase PCR as described earlier.14
Generation of DCs and transfection with rSeVs
Murine bone-marrow-derived DCs were generated as described earlier,14, 15 and an endotoxin-free condition was maintained throughout the study by using endotoxin-free reagents. Briefly, bone marrow cells from A/J mice were collected and passed through a nylon mesh, and red blood cells and lineage-positive (B220, CD5, CD11b, Gr-1, TER119, 7/4) cells were depleted by using the SpinSep mouse hematopoietic progenitor enrichment kit (StemCell Technologies, Vancouver, British Columbia, Canada). These lineage-negative cells (5–10 × 104 per 5 ml per well) were cultured in 50 ng ml−1 granulocyte-macrophage colony-stimulating factor (PeproTech, London, UK) and 25 ng ml−1 IL-4 (PeproTech) in endotoxin-free complete medium in six-well plates. On day 4, half of the culture medium was replaced by fresh medium supplemented with granulocyte-macrophage colony-stimulating factor and IL-4 at the same concentration. On day 7, DCs were collected and used for subsequent experiments. For ts-rSeV-mediated transduction, DCs (1 × 106 cells per ml) were simply incubated with rSeVs at a dose optimized earlier14, 15 and a multiplicity of infection of 100 (MOI=100) without any supplementation. At this condition, gene transduction efficiency constantly showed over 95%. Note that the DCs used in this study were not loaded tumor antigens, as was also the case earlier.15
Major histocompatibility complex class I expression on tumor cells
c1300 (mouse), SK-N-SH (human) or IMR32 (human) neuroblastoma cells (1 × 105 per ml) were incubated in the presence or absence of mouse or human IFN-β (1000 U ml−1) at 37 °C for 48 h. These cells were then stained with the corresponding fluorescein isothiocyanate-conjugated anti-major histocompatibility complex class I antibodies (mouse or human; BD Pharmingen, San Diego, CA, USA) and were analyzed using FACS Calibur (Becton Dickinson, San Jose, CA, USA) with CellQuest software (BD Biosciences Japan, Tokyo, Japan). Data analysis was performed using FlowJo 4.5 software (Tree Star, San Carlos, CA, USA). Dead cells were excluded by staining with propidium iodide.
DC-based immunotherapy to c1300 tumor
The DCs used in this study were not pulsed with any tumor antigen throughout the experiments.
‘Early’ treatment regimen
After the DCs were prepared, an immature DC phenotype appeared constantly. These immature DCs were incubated with ts-rSeV/dF-null or ts-rSeV/dF-mIFNβ for 8 h, as described earlier.14, 15 All of the DCs were added to 50 mg ml−1 of polymyxin B (Sigma-Aldrich) and were carefully washed twice before injection. Intradermal implantation (A/J for 1 × 106 of c1300 cells showing log-phase proliferation in vitro) was performed into the abdomen on day 0, and 1 × 106 DCs were injected i.t. on days 3, 10 and 17. For all injections, materials were suspended in a 100-μl volume of phosphate-buffered saline. Tumor size was assessed using microcalipers three times a week, and the volume was calculated by the following formula: tumor volume (mm3)=0.5236 × (long axis) × (short axis) × (height) (Figures 1 and 3).14, 15
‘Later’ treatment regimen and radiation pretreatment
We further assessed the ‘later treatment regimen’ for tumors that were well established, measured 7–9 mm in diameter14, 15 and constantly showed a significant vascularization histologically (data not shown). γ-Irradiation pretreatment (60Co source, 2 Gy day−1 for 3 days, daily) was performed if necessary.
Dendritic cells were collected as described above, and intradermal implantation (A/J for 5 × 105 c1300 cells) was carried out into the abdomen (Figure 1) or right thigh (Figures 3, 4 and 5: to avoid irradiation-induced enterocolitis and so on) on day 0, and 1 × 106 DCs were injected i.t. on days 10, 17 and 24. Tumor size was assessed as described above.
51Cr release assay for cytolytic activity of CTLs
Prepared DCs were i.t. administered twice into tumor-bearing mice (MH134) at 106 cells per 100 μl on days 10 and 17. One week after the last immunization, splenocytes were obtained and contaminated erythrocytes were depleted. For CTL assay, 4 × 106 splenocytes were cultured with 3 × 105 inactivated c1300 cells treated with 100 μg ml−1 mitomycin in a 24-well culture plate. Two days later, 30 IU ml−1 human rIL-2 was added to the medium. After 5 days, the cultured cells were collected and used as CTL effector cells. Target cells (c1300 cells or MuSS for third party) were labeled with 100 μCi Na251CrO4 for 1.5 h, and Cr release assay was performed as described earlier.14, 15 The percentage of specific 51Cr release of triplicates was calculated as follows: ((experimental c.p.m. × spontaneous c.p.m.)/(maximum c.p.m. × spontaneous c.p.m.)) × 100. Spontaneous release was always <10% of maximal Cr release (target cells in 1% Triton X-100).
Rechallenge of tumor cells
Nineteen animals bearing c1300 tumors were divided into five groups (Figure 6) (untreated: n=4; 4 Gy × 3 days radiation: n=3; 34 Gy × 3 days overdose radiation: n=4; ts-rSeV/dF-mIFNβ-DC: n=4; and 4 Gy × 3 days radiation+ts-rSeV/dF-mIFNβ-DC: n=4) and treated as described in the schematic regimen in Figure 4. Seven animals had survived tumor-free on day 189 (34 Gy × 3 days overdose radiation: n=3; ts-rSeV/dF-mIFNβ-DC: n=1 and 4 Gy × 3 days radiation+ts-rSeV/dF-mIFNβ-DC: n=3), and these were used for the second challenge. On day 189, 5 × 105 cells of c1300 (left) and MuSS (right) were inoculated into the bilateral dermis of the abdominal wall. Fifteen days later, tumor formation was assessed.
Statistical analysis
All data were expressed as means±s.e.m. and were analyzed by one-way analysis of variance with Fisher's adjustment, except for animal survival. Survival was plotted using Kaplan–Meier curves, and statistical relevance was determined using log-rank comparison. A probability value of P<0.05 was considered significant.
Abbreviations
- ts-rSeV:
-
temperature-sensitive mutant recombinant Sendai virus
- ts-rSeV-DC:
-
recombinant Sendai virus-modified DC
- IFN-β:
-
interferon-β
- i.t.:
-
intratumoral injection
- s.c.:
-
subcutaneous injection'
References
Henry MC, Tashjian DB, Breuer CK . Neuroblastoma update. Curr Opin Oncol 2005; 17: 19–23.
Brodeur GM . Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 2003; 3: 203–216.
Suita S, Tajiri T, Kaneko M, Hirai M, Mugishima H, Sugimoto T et al. Implications of MYCN amplification in patients with stage 4 neuroblastoma who undergo intensive chemotherapy. J Pediatr Surg 2007; 42: 489–493.
Suita S, Tajiri T, Sera Y, Takamatsu H, Mizote H, Nagasaki A et al. Improved survival for patients with advanced neuroblastoma after high-dose combined chemotherapy based in part on N-myc amplification. J Pediatr Surg 2000; 35: 1737–1741.
Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res 2001; 61: 8513–8519.
Caruso DA, Orme LM, Amor GM, Neale AM, Radcliff FJ, Downie P et al. Results of a phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with stage 4 neuroblastoma. Cancer 2005; 103: 1280–1291.
Prigione I, Corrias MV, Airoldi I, Raffaghello L, Morandi F, Bocca P et al. Immunogenicity of human neuroblastoma. Ann NY Acad Sci 2004; 1028: 69–80.
Sugio K, Nakagawara A, Sasazuki T . Association of expression between N-myc gene and MHC class I gene in surgically resected human neuroblastoma. Cancer 1991; 67: 1384–1388.
van't Veer LJ, Beijersbergen RL, Bernards R . N-myc suppresses MHC class I gene expression through down-regulation of the p50 subunit of NF-κB. EMBO J 1993; 12: 195–200.
Murphy C, Nikodem D, Howcroft K, Weissman JD, Singer DS . Active repression of major histocompatibility complex class I genes in a human neuroblastoma cell line. J Biol Chem 1996; 271: 30992–30999.
Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest 2004; 113: 1515–1525.
Rosenberg SA, Yang JC, Restifo NP . Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–915.
Banchereau J, Steinman RM . Dendritic cells and the control of immunity. Nature 1998; 392: 245–252.
Shibata S, Okano S, Yonemitsu Y, Onimaru M, Sata S, Nagata-Takeshita H et al. Induction of efficient antitumor immunity using dendritic cells activated by Sendai virus and its modulation of exogenous interferon-β gene. J Immunol 2006; 177: 3564–3576.
Yoneyama Y, Ueda Y, Akutsu Y, Matsunaga A, Shimada H, Kato T et al. Development of immunostimulatory virotherapy using non-transmissible Sendai virus-activated dendritic cells. Biochem Biophys Res Commun 2007; 355: 129–135.
Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Dian J et al. Efficient gene transfer to the airway epithelium using recombinant Sendai virus. Nat Biotechnol 2000; 18: 970–973.
Masaki I, Yonemitsu Y, Komori K, Ueno H, Nakashima Y, Nakagawa K et al. Recombinant Sendai Virus-mediated gene transfer to vasculature: a new class of efficient gene transfer vector to the vascular system. FASEB J 2001; 15: 1294–1296.
Tanaka S, Yonemitsu Y, Yoshida K, Okano S, Kondo H, Inoue M et al. Impact of deletion of envelope-related genes of recombinant Sendai viruses on immune responses following pulmonary gene transfer of neonatal mice. Gene Therapy 2007; 14: 1017–1028.
Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 2004; 5: 730–737.
Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006; 441: 101–105.
Inoue M, Tokusumi Y, Ban H, Kanaya T, Shirakura M, Tokusumi T et al. Nontransmissible virus-like particle formation by F-deficient Sendai virus is temperature sensitive and reduced by mutations in M and HN proteins. J Virol 2003; 77: 3238–3246.
Inoue M, Tokusumi Y, Ban H, Kato A, Nagai Y, Iida A et al. Further attenuation of gene(s)-deleted Sendai virus vectors: modification of transcription and replication caused weakened cytotoxicity. Mol Ther 2003; 7 (Suppl): S37 (abstract).
Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut 1998; 43: 575–577.
Nam SW, Han JY, Kim JI, Park SH, Cho SH, Han NI et al. Spontaneous regression of a large hepatocellular carcinoma with skull metastasis. J Gastroenterol Hepatol 2005; 20: 488–492.
Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58: 862–870.
Watanabe I, Kurosawa N, Nishihira T . Establishment and characterization of a murine cell-line derived from malignant fibrous histiocytoma of A/Jackson mouse. Tohoku J Exp Med 1998; 184: 173–187.
Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996; 2: 52–58.
Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4: 328–332.
Bhardwaj N . Processing and presentation of antigens by dendritic cells: implications for vaccines. Trends Mol Med 2001; 7: 388–394.
Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N . Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 2000; 191: 423–434.
Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH . Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys 1995; 33: 619–626.
Quarmby S, Kumar P, Kumar S . Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte endothelial cell interactions. Int J Cancer 1999; 82: 385–395.
Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J . The controversial abscopal effect. Cancer Treat Rev 2005; 31: 159–172.
Acknowledgements
This study was supported in part by grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to YY, no. 18390115 and to TT, no. 18591955); by Specific Educational Research Grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to TT, 2005–2008); by research grants from the Sankyo Foundation of Life Science (to YY) and from the Uehara Memorial Foundation (to YY); and by Financial Support for Cancer Research from the Children's Cancer Association of Japan (to KT, 2005–2006). We thank Miss Chie Arimatsu for her help in the animal experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
Dr Yonemitsu is a member of the Scientific Advisory Board of DNAVEC Corporation.
Rights and permissions
About this article
Cite this article
Tatsuta, K., Tanaka, S., Tajiri, T. et al. Complete elimination of established neuroblastoma by synergistic action of γ-irradiation and DCs treated with rSeV expressing interferon-β gene. Gene Ther 16, 240–251 (2009). https://doi.org/10.1038/gt.2008.161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.161
Keywords
This article is cited by
-
Oncolysis by paramyxoviruses: preclinical and clinical studies
Molecular Therapy - Oncolytics (2015)
-
Early immunisation with dendritic cells after allogeneic bone marrow transplantation elicits graft vs tumour reactivity
British Journal of Cancer (2013)
-
The effect of ionizing radiation on the homeostasis and functional integrity of murine splenic regulatory T cells
Inflammation Research (2013)
-
Gene therapy in interventional pulmonology: Interferon gene delivery with focus on thoracic malignancies
Current Respiratory Care Reports (2012)
-
Cytokine-based high log-scale expansion of functional human dendritic cells from cord-blood CD34-positive cells
Scientific Reports (2011)