Abstract
Purpose
Thoracic aortic aneurysm/aortic dissection (TAAD) is a disorder with highly variable age of onset and phenotype. We sought to determine the prevalence of pathogenic variants in TAAD-associated genes in a mixed cohort of sporadic and familial TAAD patients and identify relevant genotype–phenotype relationships.
Methods
We used a targeted polymerase chain reaction and next-generation sequencing–based panel for genetic analysis of 15 TAAD-associated genes in 1,025 unrelated TAAD cases.
Results
We identified 49 pathogenic or likely pathogenic (P/LP) variants in 47 cases (4.9% of those successfully sequenced). Almost half of the variants were in nonsyndromic cases with no known family history of aortic disease. Twenty-five variants were within FBN1 and two patients were found to harbor two P/LP variants. Presence of a related syndrome, younger age at presentation, family history of aortic disease, and involvement of the ascending aorta increased the risk of carrying a P/LP variant.
Conclusion
Given the poor prognosis of TAAD that is undiagnosed prior to acute rupture or dissection, genetic analysis of both familial and sporadic cases of TAAD will lead to new diagnoses, more informed management, and possibly reduced mortality through earlier, preclinical diagnosis in genetically determined cases and their family members.
Similar content being viewed by others
Introduction
Thoracic aortic aneurysm/aortic dissection (TAAD) manifests a devastating clinical course if undiagnosed and untreated.1 Recent studies of postmortem computerized tomographic autopsy indicate that 7% of out-of-hospital sudden deaths are due to type A aortic dissection.2
The major mortality and morbidity associated with TAAD lies with acute dissection or rupture, both of which are difficult to predict.1,3 Early perioperative mortality of a ruptured aneurysm remains high (28–46%) despite recent advances in surgical technology compared with the safety of elective treatment of unruptured thoracic aneurysms where surgical results have improved significantly (6–7% mortality).4 Aneurysm size (5–5.5 cm) is currently the major determinant of the timing of surgery.5 In syndromic aortic disease (Marfan (MFS), Loeys–Dietz (LDS), Ehlers–Danlos syndromes), earlier surgical intervention is recommended because dissection can occur at diameters less than 5.0 cm (ref.6) although these associated syndromes may be difficult to diagnose.7,8 Whilst genetic testing is recommended in familial and syndromic TAAD, it is not widely available in sporadic TAAD and is generally restricted to those with a strong suspicion of a genetic etiology.5,9
The study of large pedigrees with multiply affected members has led to the identification of causal genes in both syndromic and nonsyndromic TAAD.6,10 Causative mutations have been identified in nonsyndromic familial TAAD pedigrees within the smooth muscle contractile genes ACTA2, MYH11, PRKG1, and MYLK in nonsyndromic patients.11,12,13 Syndromic TAAD is associated with numerous connective-tissue disorders and their corresponding genes: Marfan syndrome (FBN1), Loeys–Dietz syndrome (TGFBR1, TGFBR2, SMAD3, TGFB2), Ehlers–Danlos syndrome (COL1A1, COL1A2, COL3A1, COL5A1, COL5A2), arterial tortuosity syndrome (SLC2A10), and Shprintzen–Goldberg syndrome (SKI).10,14
Many of the genes in syndromic TAAD are associated with dissections occurring in patients with an aortic size below the 5.5 cm threshold recommended for reparative surgery.6 The identification of causal variants in probands can lead to additional diagnoses by family screening in family members who have yet to develop clinical manifestations of the disorder.9 In addition, endovascular repair is contraindicated in cases diagnosed with a connective-tissue syndrome, when open surgery is recommended.15 Genetic testing can therefore identify causal variants leading to a definitive preclinical diagnosis that can better determine the optimal timing and technique of prophylactic surgery.
Next-generation sequencing is increasingly used for mutation testing and clinical diagnosis.16 Using a cost-effective polymerase chain reaction and next-generation sequencing–based targeted sequencing approach,17,18 we sought to identify the prevalence of causative genetic variants in known TAAD genes and to elucidate genotype–phenotype relationship in a mixed cohort of 1,025 cases of familial and sporadic TAAD from the United Kingdom and United States.
Materials and methods
Patient cohorts
Surgical intervention in 927 consecutive unrelated cases was undertaken over a 10-year period at the Aortic Institute at Yale–New Haven (Yale University, New Haven, CT): 785 surgical samples from these cases were used for DNA analysis within this study (Yale cohort) and 240 unrelated cases, treated and followed up at three UK centers from 2000 to 2013 (St Mary’s Hospital, London; Royal Brompton & Harefield Hospitals, London; Liverpool Heart & Chest Hospital, Liverpool) (UK cohort) were recruited to the study. Twenty-five cases within the Yale cohort were also present in a previous whole-exome sequencing study.9 The Yale cohort was approved by the Human Investigation Committee of Yale University (Institutional Review Board protocol 12617) and the UK cohort was approved by the West London Research Ethics Committee (reference 11/LO/0883). Both cohorts complied with the Declaration of Helsinki and written informed consent was obtained from all participants. MFS and LDS diagnoses in the UK cohort were identified by clinical case note review in which diagnoses were made by consultant clinical geneticists following a standard referral pathway. MFS diagnoses in the Yale cohort were made according to the Revised Ghent Nosology.19 There were no LDS cases in the Yale cohort.
DNA extraction
For the UK cohort, saliva samples were collected using the Oragene DNA kit (Genotek, Ontario, Canada), and QIAamp DNA Blood Midi kit (Qiagen, Venlo, The Netherlands) was used to extract DNA from whole-blood samples. For the Yale cohort, DNA from fresh frozen aortic specimens collected at surgery was extracted using the DNeasy Blood & Tissue kit (Qiagen, Venlo, The Netherlands). All DNA samples were subsequently normalized to 25–50 ng/μl.
Targeted exon sequencing
Targeted exon sequencing was carried out on two Fluidigm assays named aortopathy panel 1 (TAAD-X), which included 363 primer pairs for FBN1, TGFBR1, TGFBR2, MYH11, ACTA2, SMAD3, and MYLK, and aortopathy panel 2 (TAAD-Z) containing 493 primer pairs for SKI, TGFB2, SLC2A10, COL1A1, COL1A2, COL3A1, COL5A1, and COL5A2 (Supplementary Tables S1 and S2 online). Multiplex polymerase chain reaction was performed using the Access Array System (Fluidigm, South San Francisco, CA) and the MiSeq sequencing platform (Illumina, San Diego, CA) as previously described.17,18
Read mapping, variant calling, and annotation
FastQC was used to assess the sequencing read quality. Primer sequences were trimmed from FASTQ files using cutadapt (v 1.9.1)20 prior to read mapping to GRCh37/hg19 human reference sequence using BWA-MEM V0.7.12.21 Realignment of reads around indels and base quality score recalibration was performed using GATK v3.4.22 The GATK UnifiedGenotyper was used for calling variants.23 Variants were annotated using Ensembl Variant Effect Predictor v84.24
Variant and sample filtering
Synonymous variants and intronic variants (excluding splice sites) were omitted from downstream analysis unless annotated as pathogenic or disease-causing in ClinVar25 or the Human Gene Mutation Database (HGMD).26 Variants with a minor allele frequency greater than 0.1% in either ExAC release 0.3.127 or dbSNP146 28 data sets were excluded. We found a high frequency of false positives in variants within SKI exon 1 and those with an allele balance below 0.3. These variants were therefore excluded from the data sets. Samples with less than 80% of target bases covered by more than 49 reads were not considered for downstream analysis.
Pathogenicity assignment
Variants passing filters were assigned to one of four categories: either “pathogenic,” “likely pathogenic,” “variant of uncertain significance (VUS),” or “likely benign” based on the presence of one or more pieces of evidence (Supplementary Tables S3 and S4 online). Variants were assigned to the “pathogenic” or “likely pathogenic” category if they met any of the following criteria: if the variant is already reported as disease causing in HGMD or ClinVar, unless sufficient evidence could not be found for categorizing it as “pathogenic/likely pathogenic” (P/LP) (Supplementary Table S5 online); if the variant results in the same amino acid substitution as a variant already reported as disease causing in HGMD or ClinVar; premature termination of translation; a substitution of a glycine residue within a GlyXY repeat in collagen triple helical domains; an insertion of amino acids disrupting the GlyXY repeat sequence; alteration of a key residue in a protein feature (e.g., active site, disulfide bond) in keeping with previously ascribed molecular mechanisms for a given gene (see Supplementary Table S6 online). Variants predicted to be damaging by PolyPhen and SIFT and with CADD scores above 10 were assigned to the VUS category, as were variants resulting in a substitution at an amino acid position at which a different substitution is assigned disease-causing status in ClinVar or HGMD.25,26,29,30,31 Absence of PolyPhen or SIFT predictions for missense variants resulted in classification as VUS. Remaining variants were assigned “likely benign” status. All variants assigned P/LP status were validated by Sanger sequencing.
Statistical analysis
Significant differences in categorical variables between individuals in different genotype or phenotype groups were estimated by Fisher’s exact test. The unpaired Wilcoxon rank-sum test was used to assess nonparametric phenotypic continuous measurements.
Results
Patient demographics and clinical characteristics
Of the total of 785 cases recruited to the Yale cohort and 240 to the UK cohort, sequence depth reached the assay threshold of 50 reads in at least 80% of target bases in 93% of the Yale cohort and 98% of the UK cohort. A total of 732 patients in the Yale cohort and 235 patients in the UK cohort were therefore taken forward for further analyses; their demographic and clinical characteristics are given in Table 1.
Variants identified by next-generation sequencing
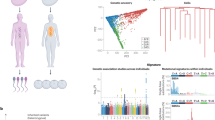
We identified 17 pathogenic variants, 32 likely pathogenic variants, and 68 VUS within the whole cohort. Two patients, Y_91_1 and Y_17_1, each carried two P/LP variants, giving a total of 49 P/LP variants in 47 patients, constituting 4.9% of the 967 samples sequenced to our target threshold (Table 2 and Supplementary Table S7 online). The majority of the identified P/LP variants were in the FBN1 gene (n = 25) (Figure 1, Supplementary Table S7 online). A total of 68 VUS were found within 67 patients (Supplementary Table S8 online).
We also tested our pipeline with a more lenient allele frequency threshold of 1%, which yielded three more P/LP variants. However, each of these additional variants were reclassified to VUS upon inspection of the literature (Supplementary Table S5 online), so a maximum allele frequency of 0.1% was used for all variant filtering.
Genotype–phenotype correlation with P/LP variants
A lower age at diagnosis was found to significantly increase the likelihood of identifying a P/LP variant. The median age at diagnosis was 46.0 years for patients carrying a P/LP variant compared with 55.5 years for those carrying a VUS (p = 3.5e−3), and 61.0 for those in the likely benign and no variant group (p = 3.4e−8) (Figure 2a). Patients who were diagnosed under 50 years of age were far more likely to carry a P/LP variant with 11.6% of those diagnosed before 50 carrying a P/LP variant compared with 3.0% in the over 50 category (p = 1.3e−7) (Figure 2b). Removing MFS cases from these data sets increases the median age at diagnosis for patients harboring a P/LP variant to 51.5 (Figure 2c) and decreases the percentage of patients under 50 harboring a P/LP variant to 7.8% (Figure 2d). The median age of TAAD diagnosis in clinically suspected MFS cases was 33.5 years in our cohort.
TAAD patients with a family history were four times more likely to carry a P/LP variant than those without a family history (p = 1.05e−4; Supplementary Figure S1a online). Gender did not significantly influence the likelihood of harboring a P/LP variant within our cohorts (p = 0.29; Supplementary Figure S1b online). A median maximum aortic size of 5.25 cm was found in patients harboring P/LP variants compared with 5.1 cm in those not harboring a P/LP variant (p = 0.45). Although aortic size is the main determinant for surgery, no association was found in our cohorts between maximum aortic size and variant categories (unpublished data). Similarly, splitting the data by pathology type and location yielded nothing significant (unpublished data).
To estimate the influence of clinical characteristics in determining the likelihood of obtaining a genetic diagnosis, we calculated the relative risk of detecting a P/LP variant by clinical phenotype (Table 3). Likelihood of genetic diagnosis was associated with presence of a related syndrome, a younger age at diagnosis, family history of aortic disease, and ascending aortic aneurysm compared with other locations. All but seven of patients harboring a P/LP variant possessed at least one of these phenotypes. Other clinical features yielded small, nonsignificant risk.
A median age at diagnosis of 39 and 29 was observed in patients who carried a P/LP variant within FBN1 (n = 25) and TGFBR2 (n = 3) respectively (Supplementary Figure S2 online). Of the 24 cases of clinically diagnosed MFS, 10 cases were identified as harboring a P/LP variant in FBN1; the remaining case (Y_35_28) in whom a presumptive genetic diagnosis was made had a likely pathogenic variant in MYH11 (Table 2). Although no pathogenic FBN1 variants were found in this patient by our panel assay, subsequent clinical exome analysis identified a probable copy-number variant in FBN1 in this patient (unpublished data), suggesting two possible genetic causes for this patient’s TAAD.
Discussion
To date, the majority of genetic abnormalities in TAAD have been identified in syndromic or familial cases.6 The aim of our study was to determine the prevalence of likely disease-causing variants in a mixed, clinically relevant cohort of patients with sporadic and familial TAAD. We sequenced 967 of 1,025 unselected TAAD patients from the United Kingdom and United States above our target coverage threshold and identified a total of 49 P/LP variants in 47 patients that are the likely cause of their disease. To our knowledge, this is the largest genetic analysis of familial and sporadic cases of TAAD to date and defines a lower limit of 4.9% for the prevalence of TAAD in our cohorts with a pathogenic abnormality among most of the currently known TAAD genes. This frequency is similar to that of deleterious mutations (3.9%) reported previously in a series of 102 TAAD patients analyzed by exome sequencing.9
The majority (87.8%) of identified P/LP variants were found within known syndromic genes. Mutations in FBN1 accounted for more than half of all identified P/LP variants, with the majority affecting functionally significant domains of the gene. P/LP variants in FBN1 were discovered in 2.6% of the sequenced cohort and 5.8% of patients who had a probable or proven family history of TAAD. This is somewhat higher than was reported in a previous study of familial TAAD, in which 2.7% of familial cases carried a pathogenic FBN1 variant.32
FBN1 is clearly an important contributor to Mendelian cases of TAAD and MFS. A recent genome-wide association study showed that common variants in FBN1 are associated with sporadic TAAD, suggesting a common pathogenesis of thoracic aortic disease in MFS and sporadic TAAD.33 We found that only 40% of those with a P/LP variant in FBN1 had a known or suspected clinical diagnosis of MFS. Similarly, only 48.9% of those with a P/LP variant in any gene had a known or probable family history of TAAD. Although these results may be partly due to an incomplete clinical record (UK records did not definitively record the absence of a family history), they are also consistent with observations over the past 20 years that FBN1 mutations are a cause of TAAD in patients who do not have clinical MFS.32,34 In keeping with this, a recent study of probands with FBN1 mutations found that only 56–79% met formal clinical criteria for MFS by Ghent systemic scores.35
Patients Y_91_1 and Y_17_1 were each found to harbor two P/LP variants (in SLC2A10 and MYH11, and FBN1 and COL1A1 respectively). No common phenotypic features (young age of onset, family history) were seen in these two patients, although a much larger maximum aortic size was found in both patients (7 cm and 6.5 cm respectively) compared with the median maximum aortic size identified in patients harboring a P/LP variant (5.25 cm).
The age at diagnosis within our patients who have a P/LP variant (46.0 years) is much lower than has been observed previously in familial TAAD (56.8 years) and sporadic TAAD (64.3 years) patients.36 This is likely due to the fact that 11 of the 47 patients harboring a P/LP variant had clinically suspected MFS, 10 of whom had P/LP variants in FBN1. The diagnosis of TAAD in MFS has been reported to have a lower age at diagnosis (24.8 years) than nonsyndromic TAAD;36 our cohort had a similarly lower age at diagnosis (33.5 years). If the patients in our cohort with suspected MFS are removed, the median age of TAAD diagnosis in our cohort rises from 46.0 to 51.5 years. Overall, the percentage of patients with a P/LP variant was 3–4 times higher within the under-50 age group than in those over the age of 50.
Although aortic size is the main determinant for surgery, we found no association between maximum aortic size and variant categories. This may in part be explained by early evaluation and surgical intervention in cases with syndromic presentations or family history of TAAD.
Comparing all 47 patients with a P/LP variant with the rest of the cohort, we were able to identify statistically significant risks of carrying a P/LP variant associated with developing the disorder: a syndromic component (risk ratio (RR) 11.94; 95% confidence interval (CI) 7.06–20.20), a younger age at presentation (RR 3.83; 95% CI 2.20–6.67), a probable or known family history of aortic disease (RR 3.80; 95% CI 1.88–7.66), and an aneurysm or dissection occurring partially or wholly within the ascending aorta (RR 1.84; 95% CI 1.04–3.27). The first three factors are clearly suggestive of Mendelian disease and may be suitable criteria for prioritizing TAAD patients for genetic testing if genetic testing is not applied to all cases. The fourth factor, disease location in the ascending aorta, is reflective of the known stronger genetic etiology of TAAD in the ascending aorta compared with the descending aorta.9
Previous studies have suggested that genetic testing should be undertaken in patients who present at a young age without any additional risk factors,37 although it is unclear to what extent this is implemented in routine clinical practice. Nonsyndromic TAAD can have a similarly severe clinical course to those with syndromic TAAD,38 highlighting the importance of identifying genetically predisposed, nonsyndromic TAAD patients prior to the development of symptoms. Seven (14.9%) patients harboring a P/LP variant did not possess any of the above phenotypes (syndromic features, young age of onset, family history, involvement of the ascending aorta) associated with greater risk of a genetic etiology. Therefore, whilst these risk factors increase the likelihood of identifying P/LP variants in known TAAD genes, restricting genetic testing solely to cases with these risk factors would miss a significant, albeit low percentage (14.9%) of cases in whom a genetic diagnosis could be made.
Our study has a number of limitations that include possibly having underestimated the number of truly pathogenic variants. We employed intentionally stringent criteria for defining P/LP variants, but this may have led to a number of truly pathogenic variants being classified as VUS. The difficulty in unequivocally designating variants as either P/LP or benign highlights the need for concerted efforts to systematically classify mutations in these disease genes, for example with prospective functional assays.39 As more information becomes available we anticipate that many of the VUS identified in this study, particularly those with strong supporting in silico data, (Supplementary Tables S8 and S9 online) may be unambiguously reclassified as causative variants. A further limitation to our study is that our sequencing assay is limited to the coding sequences and intron/exon boundaries of 15 TAAD genes that represent most of the common causes of TAAD—our study would not detect variants in as yet undiscovered genes or in noncoding sequence. In time, with greater understanding of genotype–phenotype correlation, whole-genome sequencing could ultimately detect a higher frequency of pathogenic variants than detected herein. Indeed, we were unable to identify a P/LP variant in 44 of 62 individuals with three or more of the risk factors we identified as increasing the likelihood of harboring a P/LP variant, and only 8 of these individuals were found to carry a VUS. These individuals with a high relative probability of carrying a P/LP variant but no genetic diagnosis after panel testing are likely to be worth prioritizing for future whole-genome/-exome sequencing studies.
In summary, we found 4.9% of patients carried a P/LP variant as the underlying cause of their TAAD, predominantly within FBN1 but with substantial contributions from TGFBR and COL genes. Consistent with previous reports, among these cases were patients with nonsyndromic TAAD in whom we found P/LP variants in genes normally associated with connective-tissue disease. A higher likelihood of harboring a P/LP variant was found to be associated with a syndromic component to the disease, early age at presentation, positive family history, and aneurysm location in the ascending aorta. However, restricting genetic testing only to TAAD patients with these features is likely to miss a small but significant number of cases in whom a definitive genetic diagnosis could be made.
References
Goldfinger JZ, Halperin JL, Marin ML et al. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014;64:1725–1739.
Tanaka Y, Sakata K, Sakurai Y et al. Prevalence of type A acute aortic dissection in patients with out-of-hospital cardiopulmonary arrest. Am J Cardiol 2016;117:1826–1830.
Booher AM & Eagle KA. Diagnosis and management issues in thoracic aortic aneurysm. Am Heart J 2011;162:38–46.
Goodney PP, Travis L, Lucas FL et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation 2011;124:2661–2669.
Erbel R, Aboyans V, Boileau C et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J 2014;35:2873–2926.
Milewicz DM & Regalado ES. Use of genetics for personalized management of heritable thoracic aortic disease: how do we get there? J Thorac Cardiovasc Surg 2015;149:S3–S5.
Faivre L, Collod-Beroud G, Adès L et al. The new Ghent criteria for Marfan syndrome: what do they change? Clin Genet 2012;81:433–442.
Williams JA, Loeys BL, Nwakanma LU et al. Early surgical experience with Loeys-Dietz: a new syndrome of aggressive thoracic aortic aneurysm disease. Ann Thorac Surg 2007;83:S757–S763.
Ziganshin BA, Bailey AE, Coons C et al. Routine genetic testing for thoracic aortic aneurysm and dissection in a clinical setting. Ann Thorac Surg 2015;100:1604–1612.
Lindsay ME & Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011;473:308–316.
Wang L, Guo DC, Cao J et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet 2010;87:701–707.
Guo D-C, Pannu H, Tran-Fadulu V et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007;39:1488–1493.
Guo DC, Regalado E, Casteel DE et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am J Hum Genet 2013;93:398–404.
Doyle AJ, Doyle JJ, Bessling SL et al. Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet 2012;44:1249–1254.
Grabenwöger M, Alfonso F, Bachet J et al. Thoracic endovascular aortic repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC). Eur J Cardio-thoracic Surg 2012;42:17–24.
Baker MW, Atkins AE, Cordovado SK et al. Improving newborn screening for cystic fibrosis using next-generation sequencing technology: a technical feasibility study. Genet Med 2015;18:231–238.
Weerakkody RA, Vandrovcova J, Kanonidou C et al. Targeted next-generation sequencing makes new molecular diagnoses and expands genotype-phenotype relationship in Ehlers-Danlos syndrome. Genet Med 2016;18:1119–1127.
Vandrovcova J, Thomas ERA, Atanur SS et al. The use of next-generation sequencing in clinical diagnosis of familial hypercholesterolemia. Genet Med 2013;15:948–957.
Loeys BL, Dietz HC, Braverman AC et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476–485.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011;17:10.
Li H Aligning sequence reads, clone sequences and assembly coatings with BWA-MEM. March 2013. http://arxiv.org/abs/1303.3997. Accessed 20 October 2017.
Li H & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25:1754–1760.
McKenna A, Hanna M, Banks E et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010;20:1297–1303.
McLaren W, Gil L, Hunt SE et al. The Ensembl Variant Effect Predictor. Genome Biol 2016;17:122.
Landrum MJ, Lee JM, Benson M et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res 2016;44:D862–D868.
Stenson PD, Mort M, Ball EV et al. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 2014;133:1–9.
Karczewski KJ, Weisburd B, Thomas B et al. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res 2017;45:D840–D845.
Sherry ST, Ward MH, Kholodov M et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 2001;29:308–311.
Adzhubei I, Jordan DM & Sunyaev SR Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet, Unit 7.20, 2013.
Ng PC & Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res 2003;31:3812–3814.
Kircher M, Witten DM, Jain P et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 2014;46:310–315.
Regalado ES, Guo DC, Santos-Cortez RLP et al. Pathogenic FBN1 variants in familial thoracic aortic aneurysms and dissections. Clin Genet 2016;89:719–723.
LeMaire SA, McDonald M-LN, Guo D-C et al. Genome-wide association study identifies a susceptibility locus for thoracic aortic aneurysms and aortic dissections spanning FBN1 at 15q21.1. Nat Genet 2011;43:996–1000.
Milewicz DM, Michael K, Fisher N et al. Fibrillin-1 (FBN1) mutations in patients with thoracic aortic aneurysms. Circulation 1996;94:2708–2711.
Faivre L, Collod-Beroud G, Child A et al. Contribution of molecular analyses in diagnosing Marfan syndrome and type I fibrillinopathies: an international study of 1009 probands. J Med Genet 2008;45:384–390.
Luyckx I & Loeys BL. The genetic architecture of non-syndromic thoracic aortic aneurysm. Heart 2015;101:1678–1684.
De Backer J, Renard M, Campens L et al. Genes in thoracic aortic aneurysms and dissections—do they matter? Translation and integration of research and modern genetic techniques into daily clinical practice. Aorta 2013;1:135–145.
Keramati AR, Sadeghpour A, Farahani MM, Chandok G & Mani A. The non-syndromic familial thoracic aortic aneurysms and dissections maps to 15q21 locus. BMC Med Genet 2010;11:143.
Majithia AR, Tsuda B, Agostini M et al. Prospective functional classification of all possible missense variants in PPARG. Nat Genet 2016;48:1570–1575.
Acknowledgments
The research was supported by a Medical Research Council transitional award to T.J.A., a Wellcome Trust Clinical Fellowship grant to R.W., and the National Institute for Health Research Biomedical Research Centre at Imperial College Healthcare NHS Trust and Imperial College London. We acknowledge support from Laurence Game and the London Institute of Medical Sciences Genomics Facility. We thank the patients for providing their informed consent to this research. All P/LP variants have been submitted to the ClinVar database (SUB2992095). Detailed phenotype and genotype data is available on request. Source code for data cleaning and statistical analysis can be found at https://github.com/superDross/TAAD_analysis.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosure
T.J.A. reports grants from Wellcome, grants from National Institute for Health Research, grants from UK Medical Council, personal fees from Illumina, during the conduct of the study, and personal fees from AstraZeneca, outside the submitted work. C.B. reports personal fees from Medtronic, personal fees from Bolton Medical, nonfinancial support from Vascutek, and nonfinancial support from Gore, outside the submitted work. The other authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Weerakkody, R., Ross, D., Parry, D.A. et al. Targeted genetic analysis in a large cohort of familial and sporadic cases of aneurysm or dissection of the thoracic aorta. Genet Med 20, 1414–1422 (2018). https://doi.org/10.1038/gim.2018.27
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2018.27
Keywords
This article is cited by
-
A population-based survey of FBN1 variants in Iceland reveals underdiagnosis of Marfan syndrome
European Journal of Human Genetics (2024)
-
Gendiagnostik bei kardiovaskulären Erkrankungen
Die Kardiologie (2023)
-
Analysis of the genetic contribution to thoracic aortic aneurysm or dissection in a prospective cohort of patients with familial and sporadic cases in East China
Orphanet Journal of Rare Diseases (2023)
-
Aortic gene dictionary in the precision medicine era—update from the Aortic Institute at Yale New Haven
Indian Journal of Thoracic and Cardiovascular Surgery (2022)
-
Transforming Growth Factor-β and the Renin-Angiotensin System in Syndromic Thoracic Aortic Aneurysms: Implications for Treatment
Cardiovascular Drugs and Therapy (2021)