Abstract
Purpose
To investigate the 5-year follow-up status for newborns diagnosed with metabolic disorders designated as “primary disorders” on the federal Recommended Uniform Screening Panel (RUSP).
Methods
Follow-up status and demographic characteristics are described for 426 newborns diagnosed with one of 20 primary metabolic disorders on the RUSP between 2005 and 2009. Newborn screening program data were linked to birth certificate data. Follow-up status is described for each year through age 5 and by disorder type. Maternal characteristics of those who stayed in active care were compared with those who did not.
Results
Of 426 diagnosed newborns, by the end of 5 years of follow-up 55.2% stayed in active care, 20.4% became lost to follow-up, 8.7% moved out of state, 6.3% were determined to require no further follow-up, 4.7% refused follow-up, and 4.7% died. Among the initial group of disorders with more than 10 diagnosed cases, phenylketonuria (90%) had the highest percentage of patients still in active care after 5 years. Patients in active care had similar characteristics to patients not in active care when maternal age, race/ethnicity, completed education years, and expected source of payment for delivery were compared.
Conclusion
Staying in active care may associate with disorder type but not maternal characteristics.
Similar content being viewed by others
Introduction
Over the past 10 years the value and challenges associated with providing long-term follow-up (LTFU) for children diagnosed with disorders through newborn screening (NBS) have increasingly been recognized. In 2006, the Clinical Laboratory Standards Institute described LTFU as a means to determine if the goal of NBS is being achieved through improvements in the health outcomes for affected children and their families.1 Also in 2006, Timothy Hoff2 published two papers on the practices and perception of LTFU among program staff and barriers to the collection of LTFU data.3 He concluded that eventually all state NBS programs should be involved in LTFU.2 In 2007, Hoff further addressed LTFU data collection inconsistencies across individual state NBS programs.4 His fourth paper, in 2008, described the increasing recognition of the value of LTFU in evaluating treatments and outcomes, health systems access, and quality of life for individuals with disorders that were recently added to state NBS panels, and “cultural norms” among program staff that were viewed as barriers to wider-scale adoption of LTFU data collection.5 Also in 2008, the US Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children defined LTFU as important to ensure the provision of quality chronic disease management, condition-specific treatment, and age-appropriate preventive care throughout the individual’s lifespan.6 In 2009, Botkin et al.7 described the need for systematic collection of LTFU data as a surveillance tool to address knowledge gaps and better establish the efficacy of expanded NBS.
These earlier papers set the stage for a commentary by Harvey Levy8 in a 2010 supplement of Genetics in Medicine that highlighted national efforts to incorporate LTFU into NBS programs and built on a growing consensus of the value of LTFU data for disorders detected through NBS. The special supplement included a paper by Feuchtbaum et al.9 that described the LTFU data system that was being implemented by the California NBS program. In 2011, Hinton et al.10 described LTFU data as a tool to address four overarching public health questions related to NBS care coordination, evidence-based clinical practice, continuous quality improvement, and new knowledge discovery. Hinton and colleagues also addressed health-care utilization and health outcomes for selected metabolic conditions11 in 2014 and LTFU data as a framework to assess outcomes and “the promise of newborn screening” in 2016.12
This paper expands on the 3-year follow-up data collected by California and three other states as described in the 2014 Hinton et al.11 publication. Using a 5-year cohort of California newborns diagnosed through NBS with metabolic conditions designated as primary disorders on the Recommended Uniform Screening Panel (RUSP),13 we provide comparable estimates of LTFU status by individual disorders. We also compare demographic characteristics of children who stayed in active care at a metabolic specialty care center with the group that did not stay in active care. For the children who died during the study period, we describe types of disorders and age at death.
Materials and methods
The study population included 426 newborns with confirmed primary metabolic disorders on the RUSP identified through the California Department of Public Health’s NBS Program administered by the Genetic Disease Screening Program (GDSP) from 7 July 2005 through 31 December 2009. The California NBS program is a statewide mandatory program that screens ~99% of all known live births in the state. It collects dried blood spot specimens on filter paper through a heel-stick at ≥12 hours of age (median: 30 hours) and measures acylcarnitines and amino acids using tandem mass spectrometry for metabolic disorder screening.
All newborns with initial screen-positive test results are referred to 1 of the 15 state-contracted metabolic care centers for confirmatory testing to rule out or confirm a diagnosis. Between 2005 and 2009, confirmatory testing guidelines were developed by a committee of California metabolic specialists to establish consistency in the methods used to determine a final diagnosis for different combinations of acylcarnitine and amino acid elevations. Final determination of disease status is reported to the GDSP regardless of disorder type or severity. Centers provide ongoing clinical treatment to families. Costs for services are paid either by the family’s insurance provider, MediCal (the California Medicaid program), or California Children’s Services, a program that is legislated to provide services to families without any other means of payment.
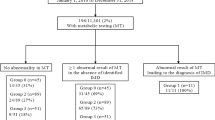
A total of 2,453,545 newborns were screened during the study period. Of the 5,859 newborns with initial positive screening results for metabolic disorders, 426 were identified with 1 of 20 primary metabolic disorders on the RUSP.13 Screening test results, diagnostic data, and clinical follow-up information for the 426 patients were reported to GDSP through a secure Web-based screening information system (SIS). The GDSP provides the metabolic care centers with a data entry manual that describes how and when data should be entered into SIS. Following an initial screen-positive result on the NBS test, short-term follow-up data is collected in SIS about the clinical diagnostic services provided to each newborn to confirm or rule out a diagnosis. For those instances where a diagnosis is confirmed, LTFU information is provided by the respective metabolic care center on a yearly basis using a Metabolic Center Annual Patient Summary (MCAPS) screen in SIS. Every day SIS updates a list for each center that displays which patients are due to have an MCAPS form completed. Follow-up centers are requested to complete the MCAPS form once a follow-up year (defined as the 1-year duration between two consecutive birthdays of each respective patient) within a month after the patient’s birthday month. The state compensates the follow-up center for each MCAPS report. A detailed description of the program’s short- and long-term follow-up data collection system has been published elsewhere.9
Center staff, usually a genetic counselor or clinic coordinator with a nursing background, enters data into SIS. Data includes a description of the cumulative medical and social services provided during the previous year of life and the health status of the child at the completion of each follow-up year. Data is collected about the laboratory tests ordered to monitor the child’s health, the type of treatment regimens initiated and the developmental status of each child at each completed year. The MCAPS also tracks the patient’s follow-up status, including whether the patient is in active care at the end of each year and the reasons for discontinued care. If the child died in the previous year, information about the date and cause of death is collected.
The MCAPS defined a patient’s follow-up status as “Active” if the patient was currently being seen at the center, as “Transferred” if the patient was transferred to another metabolic care center, as “Lost to follow-up” if whereabouts of the family were unknown and there was no way of communicating with them given that reasonable efforts to locate the family had been made and documented in SIS, as “Refused follow-up” if the patient’s parents refused follow-up, as “Determined to require no further follow-up” if further follow-up was deemed unnecessary by the metabolic care center, as “Patient not seen” if the patient was not seen in the past year but was still under active follow-up, or as “Moved out of state” or “Patient died” if the event occurred in the previous year. This analysis presented the follow-up status either as originally defined (as described above) or regrouped as “Patient in active care” (including “Active,” “Transferred,” and “Patient not seen”) and “Patients not in active care” (including “Lost to follow-up,” “Refused follow-up,” “Determined to require no further follow-up,” “Moved out of state,” and “Patient died”).
The California NBS program asks metabolic care centers to complete MCAPS forms only for patients that were in active care as reported in the MCAPS from the previous year. No further MCAPS forms were requested after a patient was reported as “Lost to follow-up,” “Refused follow-up,” “Determined to require no further follow-up,” “Patient died,” or “Moved out of state.” Patients were assigned as “Active” for a specific follow-up year with a requested but not returned MCAPS if they were “Active” in later years, and as “Lost to follow-up” if they were “Active” in the immediate previous year but did not return any other requested MCAPS forms in later years.
Patients’ follow-up status and age reported in the MCAPS forms were linked to NBS program routine data (metabolic disorder diagnosis, maternal race/ethnicity, and maternal age), California birth certificate data (maternal age, education, and expected principal source of payment for delivery), and California death certificate data (age at death) for analysis. Maternal age, based on the program routine data if available and supplemented by the birth certificate data, was categorized as <24, 25–34, and ≥35 years old. Maternal education was grouped as <12, 12, and >12 years. Expected principal source of payment for delivery was categorized as private insurance, Medi-Cal (the California Medicaid program), and other (self-pay, federal programs including CHAMPUS/TRICARE, or other). Follow-up status within a specific follow-up year and over the entire 5 years of follow-up were analyzed separately. Maternal characteristics of patients in active care were compared with those patients not in active care using chi-square testing for statistical significance.
All of the statistical analyses were conducted in SAS version 9.3 for Windows (SAS Institute, Cary, NC). The study protocol was exempted by the California Health and Human Services Agency Committee for the Protection of Human Subjects (project number 15-02-1898) as part of the ongoing program evaluation.
Results
Of the 426 patients who were referred for follow-up, 13 died within 2 months of birth so MCAPS forms were not expected. Over the 5 years of follow-up, we received 1,526 qualified MCAPS forms from the other 413 patients (94.3% of the requested 1,618 forms) completed online. Of the 92 MCAPS forms requested but not returned, about two-fifths (n = 37) were requested in the first year of follow-up. The percentage of returned MCAPS forms that were requested increased over the advance of follow-up year, with 91.1% (372/413), 92.6% (326/352), 93.5% (290/310), 97.5% (277/284), and 98.1% (254/259) in the follow-up years 1, 2, 3, 4, and 5 respectively.
The total number of patients remaining in active care dropped steadily over the cumulative 5 years of follow-up. By age 5, slightly above half (n = 235, 55.2%) of the original 426 patients remained in active care. The percentage of patients remaining in active care in a given year compared with the previous year increased in the follow-up years 2 and 3 and then stabilized in the follow-up years 4 and 5 (Figure 1). Of the 426 patients, 82.6% (n = 352) stayed in active care at age 1. The percentage went up to 88.1% (310/352), 91.6% (284/310), 91.2% (259/284), and 90.7% (235/259) in the follow-up years 2, 3, 4, and 5 respectively.
Over the cumulative 5 years, 20.4% (n = 87) of the 426 patients were lost to follow-up, 8.7% (n = 37) moved out of state, 6.3% (n = 27) were determined to require no further follow-up, 4.7% (n = 20) refused follow-up, and 4.7% (n = 20) died (Table 1). Most of the events occurred in the first year of life, including deaths (80.0%, 16/20), no further follow-up determined to be required (48.2%, 13/27), and follow-up refusals (50%, 10/20). “Patient not seen” was barely reported in the first 2 years of follow-up (n = 1 in the follow-up years 1 and 2, respectively) but more than doubled in each year of the last 3 years of follow-up (4, 9, and 18 in the follow-up years 3, 4, and 5 respectively). The proportion of those lost to follow-up in a specific follow-up year, ranging from 4.5% to 6.0%, did not change over the 5-year period.
Certain disorders were associated with a higher percentage staying in active care through age 5 (Table 2). Among the group of disorders with greater than 10 diagnosed living cases, the disorders with the highest rate of staying in active care were isovaleric acidemia (100% of 15), phenylketonuria (98.3% of 60), and medium-chain acyl-CoA dehydrogenase deficiency (MCAD, 91.9% of 111) at age 1 compared with methylmalonic academia (methylmalonyl-CoA mutase) (MUT, 72.0% of 25), carnitine uptake defect/carnitine transport defect (CUD, 67.7% of 31), and very long-chain acyl-CoA dehydrogenase deficiency (VLCAD, 77.1% of 35), which had the lowest rates of being in active care. By age 5, the highest percentage of those still in active care were phenylketonuria (90% of 60), glutaric acidemia type I (72.0% of 25), and maple syrup urine disease (62.5% of 16) with the lowest rates reported among CUD (38.7% of 31), MUT (40.0% of 25), and 3-methylcrotonyl-CoA carboxylase deficiency (3-MCC, 44.3% of 61).
Patients in active care at age 5 were not significantly different from patients not in active care in the distribution of maternal age at delivery, race/ethnicity, completed education years, and expected principal source of payment for delivery (Table 3).
A total of 20 patients died (4.7% of 426) during the 5-year follow-up. Most of them (n = 13, 65%) died within 2 months after births (Table 4). The leading disorders for the observed deaths were MUT (n = 6), MCAD (n = 4), and VLCAD (n = 3).
Discussion
Many studies have examined LTFU data to describe the clinical outcomes associated with expanded NBS using tandem mass spectrometry, including inborn errors of metabolism,14 mitochondrial fatty acid beta-oxidation defects,15 VLCAD,16,17 malonic acidemia,18 MCAD,19 and urea cycle disorders.20 Using LTFU data collected in California, Gallant21 described the biochemical, molecular, and clinical characteristics of children with short-chain acyl CoA dehydrogenase deficiency (listed as a secondary RUSP disorder) and concluded that this disorder was not associated with significant morbidity or mortality in newborns followed through age 5. Short-chain acyl CoA dehydrogenase deficiency has now been removed from the Ohio state screening panel22 and other states may be considering this option. Based on 5 years of LTFU data in California for children identified with 3-MCC, Lam et al.23 found that a significant portion of 3-MCC cases had a mild phenotype and thus 3-MCC may not be a clinically significant condition. These examples highlight the value of LTFU data in assessing the long-term clinical impact of select metabolic disorders.
In our study, the percentage of completed MCAPS forms was surprisingly high and we were able to account for the whereabouts of almost all the original cohort of cases. Close to 95% (94.3%) of requested forms were completed. More than 80% of patients (ranged from 82.6% to 91.6%) stayed in active care in the following consecutive year in each of the 5 follow-up years. We also found a very low percentage of care refusals, with less than 5% (4.7%) of patients who refused follow-up over the full 5-year period. This is a testament to the viability of the current reporting system, indicating the feasibility of conducting LTFU among patients with rare genetic disorders identified through a statewide screening program.
The percentage of children who were still in active care at age 5 ranged from 39% to 90% depending on the specific disorder. Active management at a metabolic specialty care center may provide more benefit from the parental perspective for some disorders than others. For example the high percentage of children with phenylketonuria in active care at age 5 may reflect the fact that these children require ongoing dietary management and phenylalanine monitoring, a laboratory testing service paid for by the GDSP. The disorders with the lowest percentage of patients staying in active care were 3-MCC, CUD, and MUT. The low percentage of children with 3-MCC in active care at age 5 may reflect the clinically mild impact of this disorder,24 as mentioned by Lam et al.23 Children with CUD and other conditions are possibly being managed by primary care providers in the community and therefore dropped out of active status. This leads to the recognition that not all disorders need to be managed at a metabolic specialty care center. Other possible barriers to care exist for some families, including transportation challenges when families have to travel long distances to the specialty care clinic, and language and communication barriers for non-English-speaking families. This may explain the lower number of active care patients with MUT. This disorder has been reported to be more frequent in the Hispanic population25 and therefore transportation, language, and communication challenges may disproportionately impact families of children with MUT.
Overall, the percentage of patients who were classified as lost to follow-up did not differ by age, race/ethnicity, and insurance status. These data imply that the types of patients who stayed in active care or dropped out of care was not influenced by demographic characteristics of the newborn’s family. Of the 5% of parents who specifically refused follow-up, the proportion of families who made this decision did not differ significantly by any of these demographic factors in comparison with those who stayed in active care.
The number of deaths in the cohort (20) is too small to find statistically significant differences among subgroups. The deaths seem randomly distributed by maternal characteristics, including age, race, and educational status, with one notable exception worth further study. The cases of MUT seem more concentrated in the Hispanic population, and this population seems to have a higher death rate. Evidence suggests that some of the more common variants in the Hispanic population cause more severe forms of the disease.25
The overall death rate of 4.7% is low compared with rates reported in nonscreened populations. In a French study26 that followed 187 nonscreened children with fatty acid oxidation disorders through age 5 (<6 years), the overall mortality rate was 48%. In an Australian study27 that described the clinical outcomes by age 6 years among two unscreened cohorts, the overall mortality rate was 22% (26 deaths among 116 unscreened cases, excluding phenylketonuria). We report relatively low mortality rates for VLCAD, long-chain L-3 hydroxyacyl-CoA dehydrogenase deficiency, and MCAD as 7.9%, 28.6%, and 3.5% respectively, compared with 60%, 63%, and 20% reported in the French study. The high mortality rates reported in both the French and Australian cohorts are consistent with retrospective analyses of unscreened cohorts that have an ascertainment bias caused by underdiagnosis or underreporting of milder forms of disease. The overall 4.5% mortality rate in our study is higher than the 1.7% mortality rate reported by Hinton et al.11 in 2014, which included a much smaller cohort of California cases identified in just 17 calendar months. With respect specifically to MCAD, our mortality rate of 3.5% is consistent with MCAD death rates of 4.3% and 5.1% reported from NBS in New England28 and Spain,14 respectively.
Of the 20 primary RUSP disorders included in this study, 13 are considered time-critical disorders that may present acutely in the first weeks of life and require immediate treatment to avoid death or severe disability.29 In our data, all of the deaths were among children with time-critical disorders. The majority (n = 15) occurred in the first year of life (P < 0.05, compared with the deaths beyond the first year of life). Seven of the 20 deaths (35%) occurred in the first 4 days, with 6 of the deaths being fatty acid oxidation disorders. The disorders where deaths were reported beyond 1 year included the two glutaric acidemia type I cases and three of the six MUT disorders. This data supports the importance of being able to track these children in the early years of life to more fully understand the postscreening natural history of these disorders.
Strengths of this study included successful population-based subject identification via the statewide screening program, successful longitudinal monitoring on follow-up status in each follow-up year using a large sample size of diverse metabolic disorders, and nearly complete follow-up of 5 years for each patient. The network of 15 state-contracted metabolic centers provides complete coverage of the metabolic specialty care services in California. Collection of detailed data from a limited number of specialists is logistically simpler than collecting similar data from hospitals or primary care providers.
One weakness was the potential inconsistency in data reporting from the 15 metabolic care centers. First, the final determination of disease status was made by metabolic specialists from each center. We did not evaluate how the diagnosis was made due to our policy not to override the clinicians’ diagnoses. The accuracy of the data provided in the annual patient summary based on medical chart review was also not evaluated. Another possible concern is the definition of some of the terms that captured patient status. The data entry manual that the GDSP provided gave a general definition for each follow-up status term. However, it was each center’s discretion to apply the definitions in practice. In particular, the definition of when a patient should be classified as “Lost to follow-up” might not have been uniformly applied. In addition, this study relied on existing data from MCAPS, NBS program routine data, and birth and death certificates for analysis. We were not able to examine other possible risk factors for follow-up status due to data not being available (e.g., household income and employment status).
It also must be acknowledged that a payment of approximately $200/year/patient is made to metabolic centers to provide the LTFU data described in this report. The funds to support this effort come from the NBS program fee, which was $111.70 per newborn during the study period. The annual cost of collecting LTFU data on all metabolic disorders diagnosed each year through NBS is about 1.5% of the overall revenue from the screening fees for approximately 500,000 newborns each year. Other state NBS programs may want to consider raising their NBS test fee to support the collection of LTFU data. The GDSP was able to obtain a waiver of consent by the California Committee for the Protection of Human Subjects (project 15-02-1898) allowing the collection and use of aggregate data provided by specialty care follow-up centers under contract with the state.
Future investigations on other risk factors for the lost to follow-up and parent refusals groups (respectively) may help improve the LTFU rate, including, for example, how travel distance from the family’s primary residence to the metabolic care center impacts the likelihood of staying in care. This could be important in a state as large as California, where access to care may be improved through increased use of telemedicine and/or satellite clinic availability.
Other investigations could focus on how disorder-related morbidities and health status of the child may influence a parent’s decision to discontinue, or stay in care, at a metabolic specialty care center. This phenomenon is particularly interesting for the asymptomatic child, where parents may be lulled into thinking that their child no longer needs ongoing treatment. Complicating this situation is the fact that as a result of NBS, the number of diagnosed cases is often higher than the number expected based on clinical signs and symptoms due to identification of mild biochemical phenotypes23 or genotypes with unknown clinical significance.30 Some have criticized NBS programs for causing the “unnecessary medicalization” of children,31 or the phenomenon referred to as “patients in waiting.”32 However, some asymptomatic children may be at risk for metabolic decompensation33 or neurological dysfunction later in life.34 As states move forward with adding peroxisomal and lysosomal storage diseases to their NBS panels, the value of LTFU data will become increasingly important to assess the health status of children with late-onset conditions.
Newborn screening has been described as one of the ten greatest public health achievements in the past decade.35 Despite this recognition, state NBS programs still face ongoing challenges about how to collect LTFU data to demonstrate that NBS makes a difference, and how to maximize health outcomes for children identified through this important public health program.12 Despite these challenges, if NBS is able to achieve its goals, systematic LTFU strategies will be required and this will ultimately help define the success of NBS.36
References
Clinical Laboratory Standards InstituteNewborn Screening Follow-up: Approved Guideline. Wayne, PA, 2013.
Hoff T, Hoyt A. Practices and perceptions of long-term follow-up among state newborn screening programs. Pediatrics 2006;117:1922–1929.
Hoff T, Hoyt A, Therrell B, Ayoob M. Exploring barriers to long-term follow-up in newborn screening programs. Genet Med 2006;8:563–570.
Hoff T, Ayoob M, Therrell BL. Long-term follow-up data collection and use in state newborn screening programs. Arch Pediatr Adolesc Med 2007;161:994–1000.
Hoff T. Long-term follow-up culture in state newborn screening programs. Genet Med 2008;10:396–403.
Kemper AR, Boyle CA, Aceves J et al. Long-term follow-up after diagnosis resulting from newborn screening: statement of the US Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children. Genet Med 2008;10:259–261.
Botkin JR, Anderson R, Staes C, Longo N. Developing a National Registry for conditions identifiable through newborn screening. Genet Med 2009;11:176–182.
Levy HL. Newborn screening conditions: what we know, what we do not know, and how we will know it. Genet Med 2010;12(suppl 12):S213–214.
Feuchtbaum L, Dowray S, Lorey F. The context and approach for the California newborn screening short- and long-term follow-up data system: preliminary findings. Genet Med 2010;12(suppl 12):S242–250.
Hinton CF, Feuchtbaum L, Kus CA et al. What questions should newborn screening long-term follow-up be able to answer? A statement of the US Secretary for Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children. Genet Med 2011;13:861–865.
Hinton CF, Mai CT, Nabukera SK et al. Developing a public health-tracking system for follow-up of newborn screening metabolic conditions: a four-state pilot project structure and initial findings. Genet Med 2014;16:484–490.
Hinton CF, Homer CJ, Thompson AA et al. A framework for assessing outcomes from newborn screening: on the road to measuring its promise. Mol Genet Metab 2016;118:221–229.
American College of Medical Genetics Newborn Screening Expert Group. Newborn screening: toward a uniform screening panel and system. Genet Med 2006;8(suppl 1):1S–252S.
Couce ML, Castiñeiras DE, Bóveda MD et al. Evaluation and long-term follow-up of infants with inborn errors of metabolism identified in an expanded screening programme. Mol Genet Metab 2011;104:470–475.
Baruteau J, Sachs P, Broue P et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study of 187 patients. J Inherit Metab Dis 2013;36:795–803.
Merritt JL2nd, Vedal S, Abdenur JE et al. Infants suspected to have very-long chain acyl-CoA dehydrogenase deficiency from newborn screening. Mol Genet Metab 2014;111:484–492.
Evans M, Andresen BS, Nation J, Boneh A. VLCAD deficiency: follow-up and outcome of patients diagnosed through newborn screening in Victoria. Mol Genet Metab 2016;118:282–287.
Baertling F, Mayatepek E, Thimm E et al. Malonic aciduria: long-term follow-up of new patients detected by newborn screening. Eur J Pediatr 2014;173:1719–1722.
Bentler K, Zhai S, Elsbecker SA et al. 221 newborn-screened neonates with medium-chain acyl-coenzyme A dehydrogenase deficiency: findings from the Inborn Errors of Metabolism Collaborative. Mol Genet Metab 2016;119:75–82.
Members of the Urea Cycle Disorders ConsortiumWaisbren SE Members of the Urea Cycle Disorders ConsortiumGropman AL Members of the Urea Cycle Disorders ConsortiumMembers of the Urea Cycle Disorders ConsortiumBatshaw ML. Improving long term outcomes in urea cycle disorders-report from the Urea Cycle Disorders Consortium. J Inherit Metab Dis 2016;39:573–584.
Gallant NM, Leydiker K, Tang H et al. Biochemical, molecular, and clinical characteristics of children with short chain acyl-CoA dehydrogenase deficiency detected by newborn screening in California. Mol Genet Metab 2012;106:55–61.
Ohio Administrative Code. Rule No. 3701-55-02: required screening; facility requirements – to be effective beginning 11/1/2017. In: Chapter 3701-55. Genetic, Endocrine, or Metabolic screening of newborn infants. https://www.odh.ohio.gov/en/rules/final/3701-50-59/f3701-55.
Lam C, Carter JM, Cederbaum SD et al. Analysis of cases of 3-methylcrotonyl CoA carboxylase deficiency (3-MCCD) in the California newborn screening program reported in the state database. Mol Genet Metab 2013;110:477–483.
Wilcken B. 3-Methylcrotonyl-CoA carboxylase deficiency: to screen or not to screen? J Inherit Metab Dis 2016;39:171–172.
Worgan LC, Niles K, Tirone JC et al. Spectrum of mutations in MUT methylmalonic acidemia and identification of a common Hispanic mutation and haplotype. Hum Mutat 2006;27:31–43.
Baruteau J, Sachs P, Broue P et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study from 187 patients. Complementary data. J Inherit Metab Dis 2014;37:137–139.
Wilcken B, Haas M, Joy P et al. Expanded newborn screening: outcome in screened and unscreened patients at age 6 years. Pediatrics 2009;124:e241–248.
Hsu HW, Zytkovicz TH, Comeau AM et al. Spectrum of medium-chain acyl-CoA dehydrogenase deficiency detected by newborn screening. Pediatrics 2008;121:e1108–1114.
Critical Condition Workgroup of the Society for Inherited Metabolic Disorders. SIMD Position Statement: identifying abnormal newborn screens requiring immediate notification of the health care provider. 21 August 2014. http://www.simd.org/Issues/SIMD%20NBS%20Critical%20Conditions%20policy%20statement.pdf.
Diekman E, de Sain-van der Velden M, Waterham H et al. The newborn screening paradox: sensitivity vs. overdiagnosis in VLCAD deficiency. JIMD Rep 2016;27:101–106.
Wilcken B. Expanded newborn screening: reducing harm, assessing benefit. J Inherit Metab Dis 2010;33(suppl 2):S205–210.
Timmermans S, Buchbinder M. Patients-in-waiting: living between sickness and health in the genomics era. J Health Soc Behav 2010;51:408–423.
Lindner M, Gramer G, Haege G et al. Efficacy and outcome of expanded newborn screening for metabolic diseases—report of 10 years from South-West Germany. Orphanet J Rare Dis 2011;6:44.
Kwon JM, Steiner RD. “I’m fine; I’m just waiting for my disease”: the new and growing class of presymptomatic patients. Neurology 2011;77:522–523.
Centers for Disease Control and Prevention. Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011;60:619–623.
Berry SA. Newborn screening. Clin Perinatol 2015;42:441–453.
Acknowledgments
We extend our thanks to the staff at the metabolic specialty care follow-up centers throughout California for providing the data as part of our ongoing program evaluation efforts and to the staff at our genetic disease screening laboratories who conduct the screening tests for these important diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Feuchtbaum, L., Yang, J. & Currier, R. Follow-up status during the first 5 years of life for metabolic disorders on the federal Recommended Uniform Screening Panel. Genet Med 20, 831–839 (2018). https://doi.org/10.1038/gim.2017.199
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2017.199
Keywords
This article is cited by
-
The role of exome sequencing in newborn screening for inborn errors of metabolism
Nature Medicine (2020)