Abstract
Aim:
There is increasing use of molecular technologies to guide cancer treatments, but few cost data are available. Our objective was to assess the costs of molecular-guided therapy for patients with advanced solid tumors alongside the Molecular Screening for Cancer Treatment and Optimization (MOSCATO) trial.
Materials and methods:
The study population consisted of 529 patients. The molecular diagnosis included seven steps from tumor biopsy to the multidisciplinary molecular tumor board. The cost of a complete molecular diagnosis was assessed by micro-costing. Direct costs incurred from enrollment until progression were assessed from the French National Health Insurance perspective.
Results:
The patients’ mean age was 54 years (range: 3–82) and the mean follow-up period was 145 days (range: 1–707 days). A complete molecular diagnosis cost €2,396. There were 220 patients with an actionable target (42%), among whom 105 (20%) actually received a targeted therapy. The cost of molecular-guided therapy per patient was €31,269. The main cost drivers were anticancer drugs (54%) and hospitalizations (35%).
Conclusion:
This prospective cost analysis showed that molecular diagnosis accounts for only 6% of the cost of molecular-guided therapy per patient. The costs of drugs and hospitalizations are the main cost drivers.
Genet Med advance online publication 01 December 2016
Similar content being viewed by others
Introduction
Precision medicine and molecular medicine in oncology were set as priorities in the third Cancer Plan in France.1 France is one of the leading countries using molecular diagnosis in routine practice via 28 molecular genetics centers distributed throughout the country since 2006.2 Recently, molecular profiling was implemented in clinical trials aimed at personalizing treatments.3 Numerous research projects have been conducted regarding precision medicine in oncology. This concept implies customizing patient care according to an individual’s characteristics and those of his or her own illness using either a biomarker or molecular profiling. A dozen clinical trials in oncology that use molecular profiling to guide therapeutic decision making are currently registered in the French National Agency for Medicines and Health Products Safety database (http://ansm.sante.fr).4 In these trials, different molecular profiling technologies have been used to identify key genetic aberrations that can be targeted either by approved anticancer drugs or by drugs undergoing development (phase I).
The development of precision medicine has been buoyed by the clinical success of several molecular-targeted agents linked to predictive biomarkers. The emergence of targeted therapies in oncology has created an increased need for molecular diagnosis. Next-generation sequencing (NGS) technologies significantly increased throughput speed, making it possible for DNA sequencing to garner global genomic information about patients for whom therapeutic decisions can be made expeditiously. In the past few years, the cost of sequencing has decreased exponentially (by 5 in the past 10 years),5 rendering NGS techniques affordable in clinical diagnostic settings.
Successful introduction of comprehensive molecular profiling in clinical practice relies on the development of high-throughput technologies that enable different molecular alterations to be identified in a cost-effective and timely manner.6 In this respect, two different approaches have been developed. The first is comparative genomic hybridization array (aCGH) analysis, which allows comprehensive screening of chromosome copy-number abnormalities in clinical samples. The second is targeted gene-panel sequencing (75 genes), performed with NGS platforms, that deciphers the mutational status of the main cancer genes. Altogether, this molecular profile offers information about all the types of cancer-causing alterations associated with approved anticancer drugs or drugs in development.
However, there are still obstacles that prevent us from understanding the full consequences of the clinical adoption of personalized medicine.7,8 First, there has been no clear demonstration that precision medicine is capable of enhancing the overall survival of patients with advanced cancer. Second, although the current NGS platforms run faster and are less expensive per base than previous sequencers, the cost and time required to prepare samples for sequencing and to analyze sequencing results can compromise the potential benefits. Given the huge volume of data produced by new NGS technologies, several weeks are needed to fully interpret them. This is mainly because of the large number of nucleotide variants analyzed, including rare variants with little information available to determine their pathogenic effect or their therapeutic impact as actionable features. This data-interpretation time and its associated costs are critical to clinical routine diagnosis. Accordingly, these new technologies should be assessed from both the clinical and the economic points of view. There is a strong social demand to assess the costs of molecular medicine in order to determine whether these technologies are available to and affordable for different health-care stakeholders. Many questions are being raised regarding the most appropriate methods for performing an economic assessment in this context.9,10 Previous cost studies focused on the costs of the sequencer and reagents.11 We identified a few genetic testing costs in cost-effectiveness studies of targeted therapies in oncology. However, most of them were based on public health reimbursement rates such as the Medicare fee schedule in the United States and TARMED in Switzerland12 or those directly provided by commercial laboratories.13 In these studies, cost is commonly confused with the notion of price.14 To our knowledge, no previous prospective studies have assessed the overall costs of molecular-guided therapy including both the costs of profiling technologies and the cost of treatments. Our objective was to assess the costs of molecular-guided therapy for patients with advanced solid tumors. We conducted a prospective cost study alongside the ongoing Molecular Screening for Cancer Treatment and Optimization (MOSCATO) trial.15
Materials and Methods
This paper reports the results of the economic evaluation conducted alongside the MOSCATO trial (NCT01566019).
MOSCATO trial
The MOSCATO study is a prospective, open-labeled, nonrandomized, molecular triage clinical trial conducted at Gustave Roussy, a French comprehensive cancer center. Its objective is to evaluate the feasibility of personalized medicine in patients with advanced solid tumors who experienced treatment failure. The planned accrual is 1,050 patients. After patient enrollment, ultrasound- or computed tomography–guided biopsies are performed by an interventional radiologist. The histology and the percentage of tumor cells are checked in all the samples (histological control) before any DNA extraction and molecular analysis by the genomics laboratory. High-throughput molecular techniques are used to identify genetic mutations in tumor cells as previously described.16 Briefly, aCGH analysis is performed using SurePrint G3 Human aCGH Microarray 4x180K (Agilent Technologies, Palo Alto, CA). The aCGH analysis is used mainly to detect tumor gene deletions and/or amplifications.17 Targeted NGS is performed using the Ion Torrent approach (Ion Torrent PGM; Life Technologies, Darmstadt, Germany). The libraries are generated using the Ion AmpliSeq Library kit 2.0 according to the manufacturer’s instructions (Life Technologies), starting from home-design multiplex PCR covering up to 75 target genes. Targeted NGS aims to detect any single-nucleotide variants (SNVs), including small insertion/deletion of targeted genes18 such as activating mutations in oncogenes as well as loss-of-function mutations in tumor suppressor genes. Finally, bioinformatics algorithms are used to analyze raw data, and molecular pathologists analyze the results and provide a final validated report of the molecular profile for each patient, to be used for clinical decision making.
A weekly multidisciplinary molecular tumor board (MMTB)19 convenes to optimize the management of patients with advanced and pretreated cancer who have undergone molecular testing. This MMTB, comprising oncologists, pathologists, molecular pathologists/biologists, and pharmacists, decides which treatment is best suited for each patient according to his or her molecular profile. Treatment is chosen in a defined order of preference: (i) a targeted therapy for the indication and approved by the European Medicines Agency (EMA); (ii) an experimental drug (phase I or II) targeting the identified alteration, an approved targeted therapy with temporary authorization for use, or a targeted therapy used off label; (iii) nontargeted conventional chemotherapy if no molecular abnormality is detected or if no molecular target is targetable by an existing drug.
A flowchart of the MOSCATO trial is shown in Figure 1 . The primary objective of the trial is to use high-throughput molecular profiling to improve progression-free survival compared with the previous line of treatment. Progression is defined according to RECIST (response evaluation criteria in solid tumors), clinical progression, or death from any cause. The cost analysis was a secondary objective of the trial.
Cost study
Direct medical costs were assessed from a payer perspective in the French setting. As recommended for trial-based cost analysis, we used the same time horizons for costs and outcomes. Resource use from the date of enrollment to progression was gathered in a database matching data collected within the trial on a case report form and patient-level data from Hospital Episode Statistics ( Table 1 ). All costs are expressed in 2014 euros.
The cost of molecular diagnosis. Because no reimbursement tariffs were available for molecular tests using high-throughput technologies in France at the time of our study, the cost of molecular diagnosis was assessed using micro-costing through direct observation and interviews with staff. All relevant direct cost components were valued at the most detailed level by identifying resources used directly for a patient.20 For molecular diagnosis, direct costs involved staff labor, consumables (including reagents, medical devices, and sequencing kits), equipment, and maintenance. Labor costs were valued using the time required to accomplish each task multiplied by the mean gross wage of each category of staff involved in each laboratory. Consumable costs were valued using actual consumptions and actual unit purchase prices. Equipment and maintenance costs were valued using their duration of use multiplied by their hourly cost. Hourly costs for equipment were calculated using the unit purchase price on the basis of 1,820 hours of use per year over a 5-year period. Similarly, hourly costs for maintenance were calculated using the annual contract maintenance fee divided by 1,820 hours of use per year. The overheads percentage (structure and administration costs) was obtained from the Gustave Roussy accounting system (+28% for tumor biopsy and +22% for the laboratory tests). The costs of molecular diagnosis were separated into seven steps: tumor biopsy by an interventional radiologist, dispatch of biological samples, histological control, aCGH, targeted NGS, bioinformatics analyses, and multidisciplinary molecular tumor board. The results were presented as the cost per patient, including all the diagnostic tests performed using a single sample.
The cost of hospital stays. All hospital stays at Gustave Roussy from enrollment until progression were extracted from the Hospital Episode Statistics database. The costs were computed using diagnosis-related group prices in France in 2014. Additional payments for long stays were considered, as were price cuts for short stays.
The cost of drugs
Anticancer therapies in the hospital setting. In the French prospective payment scheme, hospitals receive payment for several expensive anticancer therapies in addition to payment for the diagnosis-related groups.21 The costs of the other anticancer drugs and supportive care are included in the diagnosis-related group rates. We identified the use of expensive anticancer therapies (drug, dates, and dose) for each patient enrolled in MOSCATO using the Gustave Roussy Pharmacy database. To compute the cost of expensive drugs, we used reimbursement tariffs from the French National Health Insurance System.
Anticancer therapies in the ambulatory setting. Over the past decade, oral antineoplastic medication has changed patient management in oncology, allowing more ambulatory care.22 In addition, many anticancer targeted therapies are oral drugs. We used the French National Health Insurance drug reimbursement price list to value oral anticancer therapies administered in the ambulatory setting. We multiplied the defined daily dose (DDD) or an average dose calculated according to daily doses from the summary of product characteristics by the daily reimbursement tariff and by the treatment duration in days until progression for each patient.
Experimental drugs (phases I and II). Some of the treatments used in the trial were experimental compounds (phases I and II) whose price has not yet been defined. Nevertheless, these drugs may be commercialized in the coming years, entailing a cost burden for the health-care system. Thus, we took into account the cost of these therapies using the average daily cost of targeted therapies available on the French market since 2011 (€5,394 per month), which we applied to the treatment duration.
Medical transportation costs. Although the majority of patients enrolled in MOSCATO live in the Paris area, patients from all over France could be referred to Gustave Roussy to enter the trial. We calculated the distance between the patient’s home (zip code of residence) and Gustave Roussy. Distances for trips back and forth were valued using the tariffs from the National Health Insurance System (0.85€ per km).
Cost analysis
The cost of molecular-guided therapy was obtained by summing the cost of each component (molecular diagnosis, hospitalization, drugs, and transportation). Because some patients were not followed up at Gustave Roussy, it was not possible to collect all the resources used for these patients. The Bang and Tsiatis partitioned estimator23 was used for hospital stays and drugs to account for cost censoring (different duration of follow-up for survival and for costs) assuming that the censored patients would have had the same resource use as the patients followed up at Gustave Roussy. The distribution of the total cost and that of each cost component are reported as means and bootstrap 95% confidence intervals. Mean progression-free time was estimated as the area under the Kaplan-Meier survival curve. It represents the time horizon used for estimating costs. The heterogeneity of the cost of molecular-guided therapy was explored in a subgroup analysis considering the following: the patients with an actionable target who theoretically could have received a molecular-guided therapy (n = 220) compared with the patients without an actionable target who did not receive a molecular-guided therapy (n = 309) and the patients who actually received molecular-guided therapy according to their molecular diagnosis (n = 105) compared with the patients (n = 424) who did not receive molecular-guided therapy whatever the reason (no molecular abnormality, death before treatment initiation, no targeted treatment available for the mutations identified). Finally, a sensitivity analysis was performed excluding the cost of experimental drugs (phase I and II) to study the impact of our hypothesis for the valorization of these early therapies that have not yet been marketed.
Results
Population study
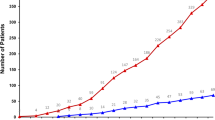
All patients enrolled in the MOSCATO trial from November 2011 to July 2014 were considered in the cost analysis. Patient characteristics are presented in Table 2 . At the time of the analysis, the study population consisted of 529 patients with advanced solid tumors. The mean age of the study population was 54 years (range: 3–82). The mean follow-up period was 145 days (range: 1–707 days). As expected, there were statistically significant differences in the cancer site between the two groups of patients with more breast cancer and lung cancer in the patient group with actionable targets (12 versus 6% for breast and 18 versus 15% for lung).
There were 220 patients with an actionable target (42%) who could have theoretically benefited from a targeted therapy. Among them, more than 93% of the patients (n = 205) had previously received chemotherapy or a targeted therapy ( Table 2 ). The patients with an actionable target received either an experimental drug (44%) or a combination of chemotherapy and another anticancer drug (25%) or chemotherapy alone (17%) or a protein kinase inhibitor (11%) or an endocrine therapy (2%) or a monoclonal antibody (1%). The patients without an actionable target received either chemotherapy (46%) or an experimental drug (21%) or a combination of chemotherapy and another anticancer drug (15%) or a protein kinase inhibitor (10%) or an endocrine therapy (6%) or a monoclonal antibody (2%). Overall, 20% (n = 105) of the patients included in the cost analysis had an actionable target and actually received targeted therapy. In these patients, targeted therapies were either experimental drugs (n = 69 patients: 66 protein kinase inhibitors, 2 monoclonal antibodies, 1 endocrine therapy) or approved anticancer drugs (n = 36 patients: 18 protein kinase inhibitors, 12 monoclonal antibodies, 3 endocrine therapies, 3 combinations of targeted therapies).
Cost analysis
Complete molecular diagnosis (micro-costing). The cost per patient of a complete molecular diagnosis obtained by micro-costing amounted to €2,396. The cost breakdown of molecular diagnosis is shown in Figure 2 . High-throughput technologies (CGH and NGS) accounted for only 40% of the total cost of molecular diagnosis. The tumor biopsy (mean duration: 75 min) and histological control represented 17 and 16%, respectively, of the cost per patient of molecular diagnosis. Molecular profiling was unsuccessful in 160 patients (30%). The failure was due to insufficient biopsy tissue (low rate of tumor cells), insufficient DNA (quantity or quality), and failed library preparation. Because the specimens of these 160 patients were not analyzed by NGS or CGH, the cost of molecular diagnosis was much lower in these patients.
Cost of molecular-guided therapy. Overall, the cost of molecular-guided therapy per patient amounted to €31,269 (95% CI: 26,181–37,765) ( Table 3 ). The main cost drivers were anticancer drugs (54%) and hospitalizations (35%). These drugs were prescribed in phase I/II trials or in daily practice. The phase I and II drugs were protein kinase inhibitors (70%), monoclonal antibodies (14%), endocrine therapies (8%), and other antineoplastic drugs (8%). Molecular diagnosis based on resource use measured in the MOSCATO trial amounted to €1,844 and represented only 6% of the cost of the molecular-guided therapy ( Table 3 ). Transportation costs accounted for 5% of the cost of the molecular-guided therapy.
Subgroup analysis. In the subgroup analysis, the cost of molecular-guided therapy per patient ranged from €29,091 (95% CI: 23,423–35,523) to €35,079 (95% CI: 28,421–42,334) ( Table 3 ). When patients with an actionable target were compared to patients without an actionable target, there was little difference in expensive anticancer drug costs. Hospitalization was the second cost driver of molecular-guided therapy (€8,682 and €11,043, respectively, in each group). For hospital stays, the main cost driver was chemotherapy administration. The cost of hospitalization was much higher (+27%) for patients without an actionable target. As a result, the cost of molecular-guided therapy was higher for these patients. By contrast, as expected, in the second comparison (targeted versus nontargeted therapy), the cost per patient of molecular-guided therapy was higher in the targeted treatment group and amounted to €35,079 (95% CI: 28,421–42,334) compared to €29,183 (95% CI: 23,000–36,315) in the nontargeted treatment group. This was explained mainly by a higher cost of expensive anticancer drugs (€22,666 versus €14,488) and particularly for the experimental (phase I/II) drugs (€14,733 versus €4,193). Within the targeted treatment subgroup, the cost of molecular-guided therapy per patient was €35,768 (95% CI: 26,907–44,711) for patients who received experimental drugs (n = 69), whereas it was €27,294 (95% CI: 24,526–39,314) for the patients who received an approved targeted therapy (n = 36). The cost of molecular diagnosis was lower for both the nontargeted treatment group (€1,707) and the nonactionable target group (€1,651) because most of these patients did not benefit from a complete molecular diagnosis in seven steps ( Figure 2 ). Mean progression-free time was not statistically significant across subgroups ( Table 3 ).
Sensitivity analysis. A sensitivity analysis was performed to determine the impact of the cost of phase I and II drugs on the overall cost of molecular-guided therapy. The cost per patient of molecular-guided therapy amounted to €23,584 (95% CI: 19,420–29,102) when phase I and II drugs were not considered (i.e., it decreased by €7,684).
Discussion
To our knowledge, this is the first study to analyze health-care costs attributable to molecular medicine in oncology conducted alongside a prospective clinical trial. This case study provided primary cost data that showed that sequencing technologies accounted for only half the cost of a complete molecular diagnosis. Incompressible costs incurred by the tumor biopsy, the dispatch of samples, the bioinformatics analysis, and the multidisciplinary therapeutic decision may hamper the expected decrease in the costs of molecular profiling. However, molecular diagnosis represented a modest part (6%) of the overall cost of molecular-guided therapy for a patient. The cost analysis revealed that drugs (phase I and II drugs and approved anticancer therapies) and hospitalizations were the main cost drivers, accounting for 54 and 35%, respectively, of the total cost per patient. Interestingly, the overall cost of treating a patient with no actionable target using standard chemotherapy was as high as the cost of treating a patient with a targeted therapy and an actionable target. This is attributable to the fact that chemotherapy implies costly hospitalizations whereas many targeted drugs are given orally in an ambulatory setting; moreover, although modern chemotherapies are less expensive than targeted drugs, they remain expensive.
This result could be seen as an advantage of molecular screening in advanced cancer patients. It is possible to imagine that in the near future more and more patients will be enrolled in molecular screening programs with new technologies that may identify actionable targets in nearly 80–90% of those patients. It will be possible to identify actionable targets such as mutations, amplifications, fusion genes, epigenetic alterations, and immunotherapy predictive biomarkers in the vast majority of patients. This improvement of molecular screening may enable discovery of potential targeted therapies for nearly every patient and may lower the cost of ineffective second- or third-line chemotherapies. Nevertheless, we acknowledge that in the current trial only 40% of the patients had an actionable target identified; therefore, one can argue that for the remaining 60%, the cost of molecular profiling was somewhat unnecessary.
Very few data exist regarding the cost and cost-effectiveness of molecular medicine.24 Using patient-level data from the Royal Marsden Cancer Centre, Slade et al.25 estimated the cost of 28 possible pathways related to breast and/or ovarian cancer and BRCA testing. In their study, the cost per patient was estimated at £2,227. Although the pathways, technologies, and cost components taken into consideration differed, our cost estimation was consistent with their finding.
Our work has several strengths. First, we used the micro-costing method to calculate the cost of tumor molecular diagnostic sequencing so that all relevant cost components were identified and valued at the most detailed level.26 Our study focused on production costs (labor, equipment, and consumables used during library preparation and sequencing), including the cost of the bioinformatics analysis. These results provide useful information that would enable the National Health Insurance System to anticipate the reimbursement of these techniques. Moreover, we estimated the cost of all the molecular-guided strategies applied (molecular diagnosis and the use of targeted therapies), based on patient-level data from a prospective clinical trial.
However, our study also had some limitations. First, some patients were not followed up at Gustave Roussy and it was not possible to collect all the information concerning them. To overcome this limitation, we used the Bang and Tsiatis methodology because patients were censored at the date of the last hospital stay for costs.23 Second, among the phases of tumor molecular diagnostic sequencing, we could not assess all upstream investments in the research and development of bioinformatics support.27 The analysis of the NGS data requires significant investment in bioinformatics to handle the huge amount of data generated.28 However, one can expect a decrease in the cost of sequencing technologies when they will be used on a larger scale. Consequently, the cost of molecular diagnosis may be slightly underestimated or overestimated in our study, which provides a snapshot of cost estimates. Third, patient characteristics (cancer site and age) might affect the generalizability of our results. However, enrollment in MOSCATO was offered to all patients likely to benefit from precision medicine. Finally, this cost study mentions nothing about the value for money of molecular medicine.29,30 Because the MOSCATO trial was noncomparative, we were unable to perform a cost-effectiveness analysis. However, the information provided here will serve as input for the future modeling of cost-effectiveness studies.
Our study shows that the cost of molecular diagnosis accounts for a very small proportion of the total cost of the strategy, especially compared to the costs of drugs. Two factors are likely to explain this finding. First, genetic sequencing costs are decreasing. The use of new technologies available for DNA molecular analysis like NGS technologies enables multiplexing of large numbers of targeted genes (including large tumor suppressor genes) and patients in the same batch of the analysis.31,32 Economies of scale reduce the cost of molecular profiling, to a certain extent, in large-scale genetic analyses. There will gradually be a transition in molecular diagnosis from testing one gene to several, many, and possibly all genes.33 Of note, almost half of the cost of molecular analysis is related to the biopsy sample and histological analysis. It is important to highlight that the tumor biopsy and tumor specimen preparation by the pathologist represent 17 and 16%, respectively, of the cost of molecular diagnosis per patient. Noninvasive biomarkers such as circulating tumor cells, cell-free DNA, and exosomes will probably be the next source of tumor material for molecular screening. These technologies need to demonstrate that the results of molecular screening will be robust, reproducible, and capable of replacing the tumor biopsy in clinical trials or in daily practice and thus will substantially decrease the cost of molecular-guided therapy per patient. These components may decrease in the future with the growing use of liquid biopsies that enable the implementation of molecular portraits using plasma samples.34
The second explanation is the high cost of targeted drugs indicated in small populations. Because cancers are driven by a number of different genetic pathways, targeted treatment may require different drug combinations for different patients. As a result, the molecular diagnosis will contribute to the establishment of niche markets by targeting patient subsets with particular genetic biomarkers. The financial model for niche markets is as follows: targeted treatments will replace blockbuster drugs, meaning that fewer patients will benefit from more effective treatments. Moreover, it is possible that genetic biomarkers, such as KRAS or NRAS mutations, will dissuade clinicians from prescribing a particular targeted therapy for colon cancer patients who do not derive a benefit from anti–epidermal growth factor receptor antibodies. This led to restriction of the use of cetuximab and panitumumab for patients with wild-type RAS tumors.35,36 Therefore, a higher price is acceptable if the health benefit is greater. Because each targeted therapy creates its own niche market, drug development strategies will evolve with a decline of the blockbuster model.
In conclusion, there is a need to generate robust economic data and establish a methodological consensus on the assessment of both the cost and the cost-effectiveness of molecular medicine.
Disclosure
The authors declare no conflict of interest.
References
Institut National du Cancer. Cancer Plan 2014–2019. Ref . PLANKPNRT14. 2015. http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Plan-Cancer-2014-2019. Accessed 7 April 2016.
Nowak F, Calvo F, Soria J-C. Europe does it better: molecular testing across a national health care system-the French example. Am Soc Clin Oncol Educ Book 2013:332–337.
Biankin AV, Piantadosi S, Hollingsworth SJ. Patient-centric trials for therapeutic development in precision oncology. Nature 2015;526:361–370.
Agence Nationale de Sécurité du Médicament et des Produits de Santé. Conduite des essais cliniques de médicaments en onco/hématologie ciblés, guidés par la génomique. 2016. http://ansm.sante.fr/var/ansm_site/storage/original/application/1c7c13b95daa7804f72df7e589867a86.pdf. Accessed 7 April 2016.
Feero WG. Genomics in medicine: maturation, but not maturity. JAMA 2013;309:1522–1524.
Normanno N, Rachiglio AM, Roma C, et al. Molecular diagnostics and personalized medicine in oncology: challenges and opportunities. J Cell Biochem 2013;114:514–524.
Tripathy D, Harnden K, Blackwell K, Robson M. Next generation sequencing and tumor mutation profiling: are we ready for routine use in the oncology clinic? BMC Med 2014;12:140.
Crawford JM, Bry L, Pfeifer J, et al. The business of genomic testing: a survey of early adopters. Genet Med 2014;16:954–961.
Annemans L, Redekop K, Payne K. Current methodological issues in the economic assessment of personalized medicine. Value Health 2013;16(6 suppl):S20–S26.
Buchanan J, Wordsworth S, Schuh A. Issues surrounding the health economic evaluation of genomic technologies. Pharmacogenomics 2013;14:1833–1847.
Blons H, Rouleau E, Charrier N, et al.; MOKAECM Collaborative Group. Performance and cost efficiency of KRAS mutation testing for metastatic colorectal cancer in routine diagnosis: the MOKAECM study, a nationwide experience. PLoS One 2013;8:e68945.
Blank PR, Moch H, Szucs TD, Schwenkglenks M. KRAS and BRAF mutation analysis in metastatic colorectal cancer: a cost-effectiveness analysis from a Swiss perspective. Clin Cancer Res 2011;17:6338–6346.
Vijayaraghavan A, Efrusy MB, Göke B, Kirchner T, Santas CC, Goldberg RM. Cost-effectiveness of KRAS testing in metastatic colorectal cancer patients in the United States and Germany. Int J Cancer 2012;131:438–445.
Buchanan J. The cost-effectiveness of next generation sequencing in colorectal cancer. Health Econ Genomics. 22 October 2015. https://healtheconomicsandgenomics.com/2015/10/22/the-cost-effectiveness-of-next-generation-sequencing-in-colorectal-cancer/.
Hollebecque A, Massard C, De Baere T, et al. Molecular screening for cancer treatment optimization (MOSCATO 01): a prospective molecular triage trial—interim results. ASCO Annual Meeting Proceedings 2013;31:2512.
Postel-Vinay S, Boursin Y, Massard C, et al. Seeking the driver in tumours with apparent normal molecular profile on comparative genomic hybridization and targeted gene panel sequencing: what is the added value of whole exome sequencing? Ann Oncol 2016;27:344–352.
Shinawi M, Cheung SW. The array CGH and its clinical applications. Drug Discov Today 2008;13:760–770.
Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet 2008;9:387–402.
Parker BA, Schwaederlé M, Scur MD, et al. Breast cancer experience of the molecular tumor board at the University of California, San Diego Moores Cancer Center. J Oncol Pract 2015;11:442–449.
Drummond MF, McGuire A. Economic Evaluation in Health Care: Merging Theory With Practice. Oxford University Press: New York, 2001.
Bonastre J, Chevalier J, Van der Laan C, Delibes M, De Pouvourville G. Access to innovation: is there a difference in the use of expensive anticancer drugs between French hospitals? Health Policy 2014;116:162–169.
Bordonaro S, Raiti F, Di Mari A, et al. Active home-based cancer treatment. J Multidiscip Healthc 2012;5:137–143.
Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87:329–343.
Christensen KD, Dukhovny D, Siebert U, Green RC. Assessing the costs and cost-effectiveness of genomic sequencing. J Pers Med 2015;5:470–486.
Slade I, Hanson H, George A, et al.; MCG programme. A cost analysis of a cancer genetic service model in the UK. J Community Genet 2016;7:185–194.
Drummond M, Sculpher M. Common methodological flaws in economic evaluations. Med Care 2005;43(7 suppl):5–14.
Sboner A, Mu XJ, Greenbaum D, Auerbach RK, Gerstein MB. The real cost of sequencing: higher than you think! Genome Biol 2011;12:125.
Desai AN, Jere A. Next-generation sequencing: ready for the clinics? Clin Genet 2012;81:503–510.
Carrera PM. Personalized medicine: worth its cost? Health Aff (Millwood) 2015;34:188.
Frueh FW. Regulation, reimbursement, and the long road of implementation of personalized medicine–a perspective from the United States. Value Health 2013;16(6 suppl):S27–S31.
Rogers YH, Venter JC. Genomics: massively parallel sequencing. Nature 2005;437:326–327.
Hert DG, Fredlake CP, Barron AE. Advantages and limitations of next-generation sequencing technologies: a comparison of electrophoresis and non-electrophoresis methods. Electrophoresis 2008;29:4618–4626.
Pasche B, Absher D. Whole-genome sequencing: a step closer to personalized medicine. JAMA 2011;305:1596–1597.
Jovelet C, Ileana E, Le Deley MC, et al. Circulating cell-free tumor DNA analysis of 50 genes by next-generation sequencing in the prospective MOSCATO trial. Clin Cancer Res 2016;22:2960–2968.
Lièvre A, Laurent-Puig P. Genetics: predictive value of KRAS mutations in chemoresistant CRC. Nat Rev Clin Oncol 2009;6:306–307.
Laurent-Puig P, Lievre A, Blons H. Mutations and response to epidermal growth factor receptor inhibitors. Clin Cancer Res 2009;15:1133–1139.
Acknowledgements
The authors are grateful to Maud Ngo Camus, Marie-Cécile Ledeley, Aljosa Celebic, Muriel Mons, Pierre Beuchet, Yann Etesse, Vincent Blazy, Yacine Rabahi, Geneviève Pétrault, Philippe Laforgue, Sébastien Roux, Romain Desmaris, Muriel Guerbert, and Amélie Gaudin, who contributed to data collection. The authors also thank the clinical team, including Antoine Hollebecque, Charles Ferté, Yohann Loriot, Sophie Postel-Vinay, Rastislav Bahleda, Anas Gazzah, Andrea Varga, and Eric Angevin, for their assistance in the enrollment of patients in the MOSCATO trial. In addition, the authors acknowledge the laboratory and bioinformatics teams, including Nathalie Auger, Sophie Cotteret, Amélie Boichard, Vladimir Lazar, Mélanie Laporte, Isabelle Miran, Nelly Motté, Ludovic Bigot, Stéphanie Coulon, Marie Petit, Catherine Richon, Aurélie Honoré, Magali Kernaleguen, Glawdys Faucher, Zsofia Balogh, Jonathan Sabio, Jean-Yves Scoazec, Philippe Vielh, Lionel Fougeat, Marie Xiberras, Leslie Girard, Lucie Herard, Catherine Lapage, Guillaume Meurice, Marc Deloger, Romy Clément Mazoyer, Chen-Min-Tao, and Yannick Boursin, for their support in the preanalytical processing of stored samples and for the analysis of tumor samples from the patients included in the MOSCATO 01 trial. Lorna Saint Ange is thanked for editing.
This cost study received financial support from the French National Agency for Research (ANR-10-IBHU-0001). The MOSCATO trial was sponsored by Gustave Roussy and received financial support from SANOFI-Aventis and Genentech.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pagès, A., Foulon, S., Zou, Z. et al. The cost of molecular-guided therapy in oncology: a prospective cost study alongside the MOSCATO trial. Genet Med 19, 683–690 (2017). https://doi.org/10.1038/gim.2016.174
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2016.174
Keywords
This article is cited by
-
Molecularly Stratified Treatment Options in Primary Refractory DLBCL/HGBL with MYC and BCL2 or BCL6 Rearrangements (HGBL, NOS with MYC/BCL6)
Targeted Oncology (2023)
-
Impact of molecular tumour board discussion on targeted therapy allocation in advanced prostate cancer
British Journal of Cancer (2022)
-
Implementation of a molecular tumor board at a regional level to improve access to targeted therapy
International Journal of Clinical Oncology (2020)