Abstract
Purpose:
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal-dominant vascular dysplasia characterized by telangiectases and arteriovenous malformations. Three causative genes are known: ENG (HHT-1), ACVRL1 (HHT-2), and SMAD4 (mutated in HHT in association with juvenile polyposis). Gastrointestinal bleeding is the most common symptom after epistaxis. The stomach and the duodenum are the main gastrointestinal sites of telangiectases. Our aim was to explore gastrointestinal tract of consecutive HHT patients to assess distribution, number, size, and type of telangiectases in relation to genotype.
Methods:
HHT patients underwent gastroduodenoscopy, video capsule endoscopy, and colonoscopy. Molecular analysis of ENG and ACVRL1 was performed to identify the disease-causing mutation.
Results:
Twenty-two patients (13 men; mean age: 59 ± 9 years) were analyzed: 7 with HHT-1, 13 with HHT-2, and 2 undefined. Gastrointestinal telangiectases were identified as follows: at gastroduodenoscopy in 86% of HHT-1 patients and in 77% of HHT-2 patients, at video capsule endoscopy in all HHT-1 patients and in 84% of HHT-2 patients, and at colonoscopy in 1 patient for each group. HHT-1 showed multiple telangiectases with a higher prevalence, more relevant in the duodenum.
Conclusion:
Our data demonstrate extensive involvement of the gastrointestinal tract with a more severe association in HHT-1. Gastroduodenoscopy provides significant information on gastrointestinal involvement, and video capsule endoscopy may be added in selected patients. Colonic polyps/adenomas were identified as occasional findings.
Genet Med 16 1, 3–10.
Similar content being viewed by others
Main
Hereditary hemorrhagic telangiectasia (HHT) or Rendu–Osler–Weber disease is a vascular dysplasia inherited as an autosomal-dominant trait.1,2 It affects ~1 in 5,000–8,000 individuals,3 with regional differences due to founder effects.4,5 The diagnosis of HHT is based on the presence of at least three of the following clinical diagnostic criteria, known as the “Curacao criteria,” established in 2000:6 (i) spontaneous, recurrent epistaxis; (ii) mucocutaneous telangiectases at characteristic sites as nose, lips, oral cavity, fingertips, and gastrointestinal (GI) mucosa; (iii) visceral arteriovenous malformations (AVMs) in lungs, liver, GI tract, brain, and spinal cord; and (iv) family history of first-degree relative in whom HHT has been diagnosed using these criteria.
Three genes whose mutations cause HHT have been identified: ENG (HHT1, #OMIM 187300);7 ACVRL1 (HHT2, #OMIM 600376);8 and, more rarely, SMAD4 (mutated in HHT in association with juvenile polyposis, #OMIM 175050),9 and many hundreds of different mutations have been described (http://arup.utah.edu/database/hht/). Molecular genetic testing (sequence analysis and multiplex ligation–dependent probe amplification or Quantitative Multiplex PCR analysis) of ENG, ACVRL1, and SMAD4 detects mutations in ~85% of individuals who meet established clinical diagnostic criteria of HHT.2 Additional as-yet-unknown HHT genes have been suggested by linkage analysis in two affected kindred on chromosome 5 and on chromosome 7.10,11 The genes mutated in HHT encode proteins that mediate signaling by the transforming growth factor-β superfamily in vascular endothelial cells.1 The mutated gene has some influence on the resultant phenotype,12,13 although extensive variations in disease expression can also be seen within the same family.14
Nose bleeding is the most common symptom, followed by GI bleeding, which occurs at the site of telangiectases in 13–30% of HHT patients. It begins commonly after the age of 50 years, is usually slow but persistent, and often becomes increasingly severe with age. It can cause significant morbidity, resulting in severe anemia and high blood transfusion requirements.15,16,17,18 Although the stomach and the duodenum have been reported to be the main GI sites of telangiectases,2,17 data concerning the involvement of the small intestine in HHT are sparse, mainly because exploration of the entire GI tract has been difficult up to now. Before our study, few papers evaluated small-bowel telangiectases in HHT patients with video capsule endoscopy (VCE), detecting telangiectases evenly distributed throughout the small bowel in 56–86% of HHT patients.19,20,21,22
The aim of this study was to use gastroduodenoscopy (EGD), capsule endoscopy, and colonoscopy (CS) to explore the entire GI tract of consecutive middle-aged or older HHT patients to assess the distribution, number, size, and type of telangiectases in relation to HHT genotype.
Materials and Methods
From October 2010 to December 2011, 22 consecutive middle-aged or older HHT patients (13 men; mean age: 59 ± 9 years; range: 46–79) identified at the Department of Otorhinolaryngology, Foundation IRCCS Policlinico San Matteo, Pavia, Italy, underwent GI exploration at the Gastrointestinal Endoscopy unit of the same hospital. Informed consent was obtained from all patients. None of the patients included had previously undergone endoscopic investigation for suspected or confirmed GI bleeding. All patients, except patient 4, had been screened for hepatic and pulmonary AVMs by color Doppler ultrasonography and computed tomography scanning, respectively. Clinical data of our cases, all diagnosed according to Curacao criteria, are summarized in Table 1 .
In addition, molecular analysis for ENG and ACVRL1 was performed to identify the disease-causing mutation at the Department of Molecular Medicine, University of Pavia. EDTA-anticoagulated blood samples from HHT patients were collected, and genomic DNA was extracted using standard procedures. Coding exons and exon–intron boundaries of ENG (Genbank NM_000118) and ACVRL1 (Genbank NM_000020) genes were amplified according to the procedure given by Olivieri et al.23 and sequenced. We also used the P093-B1 SALSA MLPA kit HHT/PPH1 (MRC-Holland, Amsterdam, The Netherlands) to analyze large deletions or duplications in ACVRL1, ENG, and BMPR2 genes according to the manufacturer’s instructions.
To demonstrate the presence of GI telangiectases, all 22 patients were subjected to EGD and VCE, and only 17 also underwent CS. In four cases, CS was not performed due to refusal or poor general conditions and in one patient, it was performed in a different hospital as a follow-up for a rectal spinocellular carcinoma, 15 days before admission to our hospital. The ethics committee of the Foundation IRCCS Policlinico San Matteo approved the study.
EGD was carried out by EVIS EXERA II, GIF-Q165, (Olympus, Tokyo, Japan), after an overnight fast of 12 h. VCE was performed using a wireless capsule (M2A; Given Imaging, Yoqneam, Israel). Before the procedure, a clear fluid diet was recommended for 24 h, and the patients took 2 l of polyethylene glycol (140 g) 16 h before VCE.
The telangiectases were classified according to number: few (≤10) or multiple (>10); size: small (≤5 mm) or large (>5 mm); and type following the Yano–Yamamoto classification,24 which classifies lesions into the following six groups: type 1a, punctulate erythema (<1 mm), with or without oozing; type 1b, patchy erythema (a few mm), with or without oozing; type 2a, punctulate lesions (<1 mm), with pulsatile bleeding; type 2b, pulsatile red protrusion, without surrounding venous dilatation; type 3, pulsatile red protrusion, with surrounding venous dilatation; type 4, other lesions not classified into any of the above categories. Types 1a and 1b are considered angioectasias. Types 2a and 2b are Dieulafoy’s lesions. Type 3 represents an arteriovenous malformation. Type 4 is unclassifiable ( Figure 1 ).
Statistical analysis
HHT1 and HHT2 groups were compared using Student’s t-test for continuous data and the χ2 test for categorical data. P values of <0.05 were considered to be statistically significant.
Results
Twenty-two consecutive middle-aged or older HHT patients (13 men; mean age: 59 ± 9 years; range: 46–79) were analyzed.
Molecular analysis
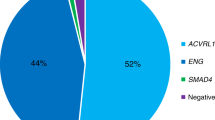
Seven patients carried ENG mutations (HHT1) and 13 carried ACVRL1 mutations (HHT2), in agreement to the relative proportions of the two forms in Italy.23 Five mutations are as yet unpublished (patients 2, 11, 15, 17, and 19 in Table 2 ), and one of these is a duplication of exons 5, 6, and 7 of ENG, analyzed by multiplex ligation–dependent probe amplification. Moreover, in two patients, the disease-causing mutation is still unknown. No significant differences were found between the two groups of HHT patients regarding age, sex, or hemoglobin (Hb) levels ( Table 1 ).
Endoscopic evaluation
Telangiectases were found throughout the digestive tract in 20 of 22 (91%) patients, at EGD in 18 of 22 (82%) patients, at VCE in 20 of 22 (91%) patients, and at CS in 2 of 17 (12%) patients.
EGD: In Table 2 , we report the number and size of telangiectases identified by EGD in the proximal tract of HHT patients. In four cases (patients 4, 10, 16, and 21), telangiectases were present only in the duodenum. There is a higher prevalence of telangiectases in HHT1 than in HHT2 (P = 0.6) ( Figure 2a ). The differences become statistically significant when the number of telangiectases is considered; HHT1 cases show, in fact, more frequent multiple telangiectases than HHT2 cases (P < 0.02), both in stomach and in the duodenum ( Figure 2b , c ).
Box and whisper plots summary of telangiectases distribution in GI tract. For each item, the bar on the left relates to HHT1 patients. (a) Age in HHT1 and HHT2 patients with (+) and without (−) proximal tract involvement at EGD. (b) Age in HHT1 and HHT2 patients with telangiectases in the stomach: number versus size. (c) Age in HHT1 and HHT2 patients with telangiectases in the duodenum: number versus size. (d) Age in HHT1 and HHT2 patients with (+) and without (−) proximal tract involvement at VCE. (e) Age in HHT1 and HHT2 patients with telangiectases in the duodenum: number versus size. (f) Age in HHT1 and HHT2 patients with telangiectases in the jejunum: number versus size. (g) Age in HHT1 and HHT2 patients with telangiectases in the ileum: number versus size. EGD, gastroduodenoscopy; HHT, hereditary hemorrhagic telangiectasia; VCE, video capsule endoscopy.
VCE: All patients were subject to VCE without complications. The average gastric transit time was 50 min (range: 3–180 min), and the average small-bowel transit time (interval between the first image from the duodenum and the first image from the colon) was 3 h and 52 min (range: 2.34–5.5 h). In Table 2 , we entered the results of VCE imaging, reporting, for all segments examined, the number, size, and type of telangiectases according to the Yano-Yamamoto classification. The percentage of patients positive for telangiectases is higher for HHT1 patients than for HHT2 patients (P < 0.3) ( Figure 2d ), and the difference became significant when the number and size of telangiectases were considered (P < 0.02). The duodenum is largely involved at VCE also, with HHT1 patients more frequently affected; this result is also observed for the jejunum (P < 0.05). The difference between HHT1 and HHT2 patients is significant when both the number and the size of telangiectases are considered in the duodenum (P < 0.01 and P < 0.05, respectively) ( Figure 2e ) and when only size is considered in the jejunum (P < 0.05) ( Figure 2f ). The ileum is slightly less involved for HHT1 cases, but as compared with the duodenum and jejunum, the number of large telangiectases is higher in both HHT1 and HHT2 cases ( Figure 2g ). Active bleeding ( Table 2 ) was observed only in the jejunum in patients 7 and 14; both of them had very low levels of Hb: a blood transfusion was requested for patient 7 shortly before GI examinations, and the Hb level of patient 14 was 3.9 g/dl. No other abnormalities, in addition to telangiectases, were found in the proximal intestinal tract, except in patient 19, who had multiple erosions in the terminal ileum compatible with inflammatory bowel disease and is still under evaluation.
CS: Seventeen patients underwent CS. Telangiectases were identified in 2 of 17 patients (12%, one each for HHT1 and HHT2). In both cases, telangiectases were few and small. We identified polyps in 3 of 17 HHT (18%) patients, adenoma in 1 (6%), and hyperplastic polyps in 2 (12%), which were removed by endoscopic polypectomy.
Other clinical manifestations
General information about clinical presentation of patients is summarized in Table 1 .
We identified pulmonary AVMs in 9 of 21 and hepatic AVMs in 11 of 21 HHT patients. As expected, we observed differences in the incidence of pulmonary AVMs (higher in HHT1, P < 0.02) and of hepatic AVMs (higher in HHT2, P < 0.3). The mean Hb level was 10.38 ± 2.61 g/dl (range: 3.9–15.3 g/dl). Six (27%) patients had anemia, defined as a Hb level of ≤9 g/dl, not correlated to the frequency and intensity of nosebleeds; five (83%) of them had, in effect, multiple telangiectases in the small bowel, one with active bleeding in the jejunum. Patient 9 (Hb: 12.1 g/dl) required a blood transfusion 1 week before GI examination.
Discussion
Angiodysplasia in the GI tract can be found in up to 3% of the population. It is usually asymptomatic but may sometimes result in severe bleeding. This type of lesion is probably responsible for ~6.0% of cases of lower GI bleeding and 1.2–8.0% of cases of hemorrhage from the upper GI tract.25 GI telangiectases affect 13–30% of HHT patients. These lesions may cause severe bleeding usually after the fifth or sixth decade of life and lead to significant anemia requiring blood transfusions.15,16,17,18 GI telangiectases frequently pose difficult problems in diagnosis and treatment, and EGD and CS are the most commonly used diagnostic tools. The lesions in the small intestine, however, are inaccessible to conventional methods, and until a few years ago, they were identified only by invasive practices such as push enteroscopy and more recently double-balloon enteroscopy. The recent introduction of VCE makes it possible to explore the entire small bowel, safely and noninvasively, and provides excellent images of this tract.26 In the current study, we explored the entire GI tract to determine the distribution and prevalence of telangiectases in the stomach, duodenum, jejunum, ileum, and colon in a group of unselected HHT patients in relation to their genotype.
Few previous studies report details about the prevalence and distribution of telangiectases in the GI tract of HHT patients. Ingrosso et al.19 performed EGD and VCE in 20 consecutive HHT patients. The prevalence was 75% in the gastric tract and 56% in the small bowel, with a preferential occurrence in older patients. Proctor et al.,27 using an enteroscope in 27 unselected HHT patients, found at least one telangiectasia in stomach/duodenum in all cases and also noted a strong correlation between the number of telangiectases in the stomach and duodenum as compared with the number in the jejunum. Chamberlain et al.20 compared a group of 38 consecutive HHT patients with a group of patients without HHT. VCE detected telangiectases evenly distributed throughout the small bowel in 81% of HHT patients versus 29% in non-HHT cases. The genotype is not taken into account in any of these reports. van Tuyl et al.21 completed molecular analysis of ENG and ACVRL1 in 25 HHT patients selected on the basis of severe anemia; EGD revealed telangiectases in 67%, VCE in 76%, and CS in 32% of HHT patients. They found a higher prevalence of large telangiectases in HHT1 patients older than 50 years. Grève et al.22 detected gastric and small-bowel telangiectases with VCE in 46.7 and 86.7% of 30 cases with HHT and severe anemia. Their population was essentially composed of patients with the ACVRL1 mutation. Although it is not easy to compare the results reported in the previously quoted papers, we can observe that the percentage of patients with gastric telangiectases is most likely between 46 and 75%, whereas lesions found in small bowel may be slightly more frequent (56–86%).
In our study, we found an incidence comparable to those of previous reports for the stomach (64%) and slightly higher for the small bowel (91%). As to age, in agreement with Ingrosso et al.19 and van Tuyl et al.,21 we found a higher incidence of telangiectases in patients older than 50 years, in both HHT1 and HHT2 ( Figure 2 ).
Kjeldsen and Kjeldsen17 observed more severe GI bleeding and, consequently, more frequent transfusions in HHT1 patients as compared with HHT2 patients; van Tuyl et al.21 confirmed these data and reported that the prevalence of telangiectases in small bowel (assessed by VCE) was significantly higher in patients with HHT1 than that in patients with HHT2 (100 vs. 63%).
In our study, at VCE, we found telangiectases in all HHT1 patients and in 84% of HHT2 patients. Furthermore, we found a significantly higher rate of multiple telangiectases in the small intestine of HHT1 versus HHT2 patients, mainly due to the difference observed in the duodenum (P < 0.01) ( Figure 2e ), also underlining some phenotypical differences between HHT1 and HHT2 with respect to GI localization.
We discussed genotype–phenotype correlation in terms of HHT1 vs. HHT2 because it is generally accepted that haploinsufficiency for either gene will cause the disease. By this mechanism, we do not expect single mutations to show consistent differences at the phenotypic level. In Table 2 , in patients 1 and 8, no GI lesions were observed, but both patients had hepatic AVMs and epistaxis. Both patients carry ACVRL1 mutations in exon 3, an in-frame deletion (p. L55_V56del), and a missense mutation (p. C51Y). The missense mutation was included in the paper by Scotti et al.28 and was among the mutations with the higher destabilizing effects on protein structure. The presence of hepatic AVMs fits with its increased frequency in females carrying ACVRL1 mutations;13 both cases are younger than those more severely affected, and, currently, younger age is likely a better explanation for their mild GI presentation of the disease than the presence of a peculiar mutation.
Our paper is the first in which clinical data are linked to the mutations found in each single patient, and discussion on the effect of single mutations on GI phenotype in HHT should be postponed until a larger amount of comparable data is available.
We observe that the most frequent condition in our patients is the simultaneous presence of telangiectases in the stomach and small intestine; EGD was able to identify telangiectases in stomach and/or duodenum in 64% of patients, whereas VCE identified telangiectases in 91% of patients. It is of relevance that we observed concordance of the results obtained for duodenum by EGD and VCE in all cases but one: in this patient (patient 19), EGD failed to demonstrate any telangiectases in the stomach or in the duodenum, whereas few and small duodenal lesions were observed by VCE.
EGD did not show gastric telangiectasia in eight patients (seven HHT2; mean Hb: 10.95 g/dl). Among them, four patients, however, showed few and small telangiectasia in the duodenum, confirmed by VCE in three of four. Among the three in whom no lesions were identified in the stomach and duodenum with both EGD and VCE, two did not show any lesions either in the distal portion of the small intestine, whereas the third showed only few and small lesions likely of minor clinical relevance. Therefore, it seems that negative findings by EGD may forecast absence or minimal involvement also in the small intestine. Patient 19 (HHT1) was negative by EGD but positive by VCE in the small intestine, but the number of lesions was ≤10, and the size was small and the type was 1a ( Table 2 ), so the differences with other EGD-negative cases are minimal.
For the description of small-bowel vascular lesions, we adopted Yano-Yamamoto classification,24 which considers strictly telangiectases (venus/capillary lesions that consist of thin tortuous veins that have no internal elastic layer) types 1a and 1b. These types are easily recognizable by VCE, whereas it is difficult to see the typical pulsation of the type 3. The vast majority of lesions were type 1b, irrespective of the gene involved. Telangiectases type 3 (pulsatile red protrusion, with surrounding venous dilatation) were observed in only HHT2 patients ( Table 2 ). In our experience, the use of this classification proved useful for a homogeneous description of patients.
It is now generally agreed that telangiectases in HHT patients are more probably found in the stomach and duodenum (ref. 19, see Results). EGD can easily analyze these portions of the GI tract; the additional use of VCE can be suggested, as it provides better information for the distal part of duodenum and for the small intestine in general in patients in whom there is a higher probability of larger number of lesions (HHT1, older age, and severe anemia with limited nosebleed). In these selected cases, a less invasive method such as VCE may be a better choice. In addition, the extensive mapping of lesions obtained by VCE is preliminary to treatment of large lesions, for instance, with argon plasma coagulation and double-balloon enteroscopy. All patients in whom EGD found telangiectases were treated without complication with argon plasma coagulation. Patient 17 was not treated because of very mild involvement, whereas in patient 2 (HHT1), argon plasma coagulation was not applied due to the very large number of lesions (>100). Therefore, some cases require additional or alternative therapy to the coagulation (as double-balloon enteroscopy), mainly when transfusion requirements are extensive and lesions are multiple and located proximally in the small bowel. Therefore, pharmacological therapies with estrogen/progesterone and the somatostatin analog octreotide have been considered as adjunctive measures in these patients. The results are not uniformly successful, and further studies are warranted. The most recent addition to the list of pharmacological agents reported to be useful for bleeding angiodysplasia is thalidomide. In high doses (400 mg/day), it has anti–tumor necrosis factor effects, whereas in lower doses (100–200 mg/day), it has antiangiogenetic effects. Patients improved in as little as 2 weeks, and the effect was sustained for a mean of 33 months.29
Forsberg et al.30 recently reported prevalence data about colonic lesions in the normal population: one or more polyps were found in 27% of individuals, whereas at least one adenoma was found in 8% of cases, and patients in whom adenoma was found were significantly older than those with a negative CS. On the basis of similar evidence previously published, “European guidelines for quality assurance in colorectal cancer screening and diagnosis” suggest fecal occult blood test for colorectal cancer screening starting from 54 years. In HHT patients (irrespective of their age), as epistaxis is present in more than 90% of cases, fecal occult blood test cannot be used as the first screening test, and CS becomes the most relevant test for colorectal cancer screening.31 Our data confirmed that the colon is less involved for the presence of telangiectases (only 2 of 15 cases showing few and small telangiectases), and occasional finding of polyps and adenomas (13 and 7%, respectively) is comparable to the expected incidence for the general population, if age is taken into account. Similar data were obtained for CS reported in HHT20,22 with 4 of 58 positive cases (7%).
Disclosure
The authors declare no conflict of interest.
References
Shovlin CL . Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev 2010;24:203–219.
McDonald J, Bayrak-Toydemir P, Pyeritz RE . Hereditary hemorrhagic telangiectasia: an overview of diagnosis, management, and pathogenesis. Genet Med 2011;13:607–616.
Faughnan ME, Palda VA, Garcia-Tsao G, et al.; HHT Foundation International – Guidelines Working Group. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011;48:73–87.
Lesca G, Genin E, Blachier C, et al.; French-Italian HHT Network. Hereditary hemorrhagic telangiectasia: evidence for regional founder effects of ACVRL1 mutations in French and Italian patients. Eur J Hum Genet 2008;16:742–749.
Westermann CJ, Rosina AF, De Vries V, de Coteau PA . The prevalence and manifestations of hereditary hemorrhagic telangiectasia in the Afro-Caribbean population of the Netherlands Antilles: a family screening. Am J Med Genet A 2003;116A:324–328.
Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000;91:66–67.
McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 1994;8:345–351.
Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 1996;13:189–195.
Gallione CJ, Repetto GM, Legius E, et al. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 2004;363:852–859.
Cole SG, Begbie ME, Wallace GM, Shovlin CL . A new locus for hereditary haemorrhagic telangiectasia (HHT3) maps to chromosome 5. J Med Genet 2005;42:577–582.
Bayrak-Toydemir P, McDonald J, Akarsu N, et al. A fourth locus for hereditary hemorrhagic telangiectasia maps to chromosome 7. Am J Med Genet A 2006;140:2155–2162.
Kjeldsen AD, Møller TR, Brusgaard K, Vase P, Andersen PE . Clinical symptoms according to genotype amongst patients with hereditary haemorrhagic telangiectasia. J Intern Med 2005;258:349–355.
Lesca G, Olivieri C, Burnichon N, et al.; French-Italian-Rendu-Osler Network. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet Med 2007;9:14–22.
Govani FS, Shovlin CL . Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet 2009;17:860–871.
Smith CR Jr, Bartholomew LG, Cain JC . Hereditary hemorrhagic telangiectasia and gastrointestinal hemorrhage. Gastroenterology 1963;44:1–6.
Vase P, Grove O . Gastrointestinal lesions in hereditary hemorrhagic telangiectasia. Gastroenterology 1986;91:1079–1083.
Kjeldsen AD, Kjeldsen J . Gastrointestinal bleeding in patients with hereditary hemorrhagic telangiectasia. Am J Gastroenterol 2000;95:415–418.
Longacre AV, Gross CP, Gallitelli M, Henderson KJ, White RI Jr, Proctor DD . Diagnosis and management of gastrointestinal bleeding in patients with hereditary hemorrhagic telangiectasia. Am J Gastroenterol 2003;98:59–65.
Ingrosso M, Sabbà C, Pisani A, et al. Evidence of small-bowel involvement in hereditary hemorrhagic telangiectasia: a capsule-endoscopic study. Endoscopy 2004;36:1074–1079.
Chamberlain SM, Patel J, Carter Balart J, Gossage JR Jr, Sridhar S . Evaluation of patients with hereditary hemorrhagic telangiectasia with video capsule endoscopy: a single-center prospective study. Endoscopy 2007;39:516–520.
van Tuyl SA, Letteboer TG, Rogge-Wolf C, et al. Assessment of intestinal vascular malformations in patients with hereditary hemorrhagic teleangiectasia and anemia. Eur J Gastroenterol Hepatol 2007;19:153–158.
Grève E, Moussata D, Gaudin JL, et al. High diagnostic and clinical impact of small-bowel capsule endoscopy in patients with hereditary hemorrhagic telangiectasia with overt digestive bleeding and/or severe anemia. Gastrointest Endosc 2010;71:760–767.
Olivieri C, Pagella F, Semino L, et al. Analysis of ENG and ACVRL1 genes in 137 HHT Italian families identifies 76 different mutations (24 novel). Comparison with other European studies. J Hum Genet 2007;52:820–829.
Yano T, Yamamoto H . Vascular, polypoid, and other lesions of the small bowel. Best Pract Res Clin Gastroenterol 2009;23:61–74.
Foutch PG . Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol 1993;88:807–818.
Iddan GJ, Swain CP . History and development of capsule endoscopy. Gastrointest Endosc Clin N Am 2004;14:1–9.
Proctor DD, Henderson KJ, Dziura JD, Longacre AV, White RI Jr . Enteroscopic evaluation of the gastrointestinal tract in symptomatic patients with hereditary hemorrhagic telangiectasia. J Clin Gastroenterol 2005;39:115–119.
Scotti C, Olivieri C, Boeri L, et al. Bioinformatic analysis of pathogenic missense mutations of activin receptor like kinase 1 ectodomain. PLoS ONE 2011;6:e26431.
Szilagyi A, Ghali MP . Pharmacological therapy of vascular malformations of the gastrointestinal tract. Can J Gastroenterol 2006;20:171–178.
Forsberg AM, Kjellström L, Agréus L, et al. Prevalence of colonic neoplasia and advanced lesions in the normal population: a prospective population-based colonoscopy study. Scand J Gastroenterol 2012;47:184–190.
Elinav E, Salameh-Giryes S, Ackerman Z, Goldschmidt N, Nissan A, Chajek-Shaul T . Does any lower gastrointestinal bleeding in patients suffering from hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu) necessitate a full colonic visualization? Int J Colorectal Dis 2004;19:595–598.
Acknowledgements
We thank the patients and their families for their cooperation and the association “Fondazione Italiana HHT-Onlus Onilde Carini” for its support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canzonieri, C., Centenara, L., Ornati, F. et al. Endoscopic evaluation of gastrointestinal tract in patients with hereditary hemorrhagic telangiectasia and correlation with their genotypes. Genet Med 16, 3–10 (2014). https://doi.org/10.1038/gim.2013.62
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2013.62
Keywords
This article is cited by
-
Predictors of mortality in patients with hereditary hemorrhagic telangiectasia
Orphanet Journal of Rare Diseases (2021)
-
Mutational and clinical spectrum of Japanese patients with hereditary hemorrhagic telangiectasia
BMC Medical Genomics (2021)
-
A case report of hereditary hemorrhagic telangiectasia in a family with initial presentation of cerebral abscess and pulmonary arteriovenous malformation in the proband
Neurological Sciences (2020)
-
Rendu-Osler-Weber disease: a gastroenterologist’s perspective
Orphanet Journal of Rare Diseases (2019)
-
Abdominal manifestations of hereditary hemorrhagic telangiectasia: a series of 333 patients over 15 years
Abdominal Radiology (2019)