Abstract
Purpose: To estimate the cost-effectiveness of genetic testing strategies to identify Lynch syndrome among newly diagnosed patients with colorectal cancer and to offer targeted testing to relatives of patients with Lynch syndrome.
Methods: We calculated incremental costs per life-year saved for universal testing relative to no testing and age-targeted testing for strategies that use preliminary genetic tests (immunohistochemistry or microsatellite instability) of tumors followed by sequencing of mismatch repair genes. We also calculated incremental cost-effectiveness ratios for pairs of testing strategies.
Results: Strategies to test for Lynch syndrome in newly diagnosed colorectal tumors using preliminary tests before gene sequencing have incremental cost-effectiveness ratios of ≤$45,000 per life-year saved compared with no testing and ≤$75,000 per life-year saved compared with testing restricted to patients younger than 50 years. The lowest cost testing strategies, using immunohistochemistry as a preliminary test, cost ≤$25,000 per life-year saved relative to no testing and ≤$40,000 per life-year saved relative to testing only patients younger than 50 years. Other testing strategies have incremental cost-effectiveness ratios ≥$700,000 per life-year saved relative to the lowest cost strategies. Increasing the number of relatives tested would improve cost-effectiveness.
Conclusion: Laboratory-based strategies using preliminary tests seem cost-effective from the US health care system perspective. Universal testing detects nearly twice as many cases of Lynch syndrome as targeting younger patients and has an incremental cost-effectiveness ratio comparable with other preventive services. This finding provides support for a recent US recommendation to offer testing for Lynch syndrome to all newly diagnosed patients with colorectal cancer.
Similar content being viewed by others
Main
Lynch syndrome is a genetic predisposition to colorectal cancer (CRC) and certain other malignancies as a result of a germline mismatch repair (MMR) gene mutation. In this article, we present an economic evaluation of genetic testing protocols to identify Lynch syndrome among newly diagnosed cases of CRC in order to identify and test blood relatives for the presence of Lynch syndrome. The benefit of identifying an asymptomatic individual with Lynch syndrome is that it allows for early and intensive surveillance to detect colon polyps, which can prevent malignancies and reduce the risk of premature death.
The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (EWG) commissioned evidence reviews1,2 of testing strategies for Lynch syndrome and in January 2009 published a recommendation to offer laboratory testing to all newly diagnosed patients with CRC, regardless of age or family history.3 The primary research question was whether the EWG recommendation of universal testing to identify mutations for which close relatives could be screened was economically justified from a national health care system perspective. The secondary research question was which laboratory testing strategies to identify mutations associated with Lynch syndrome could be regarded as providing reasonable value for money. This article extends a preliminary analysis of the expected costs of four testing strategies, and the numbers of new CRC cases identified with Lynch syndrome2 to include the costs and lives saved from screening asymptomatic first-degree relatives of newly diagnosed Lynch syndrome patients followed by colonoscopic surveillance. Because of uncertainty about the availability, feasibility, and accuracy of the laboratory tests, our goal was to inform health care providers, laboratory directors, and policy makers about the costs and outcomes associated with each testing strategy.
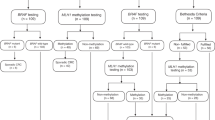
Although a number of groups recommend targeted offering of testing for Lynch syndrome based on either a diagnosis age <50 years or having a family history that meets the Amsterdam or Bethesda criteria,4–6 the EWG argued that the collection and interpretation of family history information for this purpose is not of sufficient reliability to be used in routine clinical practice.2,3 Although most European countries, with the exception of Denmark, recommend the use of Bethesda family history criteria to guide genetic testing for Lynch syndrome, the quality of family history data collection is regarded as variable to poor.7 To more fully evaluate the cost-effectiveness of testing for Lynch syndrome, we consider both universal testing, i.e., offer to test all newly diagnosed patients with CRC for Lynch syndrome and testing only those diagnosed while <50 years of age (age-targeted testing).
METHODS
Overview
We developed a decision model to estimate the cost-effectiveness of a population-based testing program for Lynch syndrome from the US health care system perspective, using estimates of direct costs of screening, diagnosis, and health care associated with CRC. The model estimates total costs for the US health care system as a whole rather than expenditures incurred by specific payers. The primary audience for our study consists of public and private health decision makers. Laboratory directors are a secondary audience because health care payers and physicians do not necessarily decide which specific laboratory tests will be used.
The proposed program has three components: (1) detecting probands with Lynch syndrome through laboratory testing of tissue from newly diagnosed patients with CRC, (2) offering testing for Lynch syndrome to first-degree relatives of probands using the identified family mutation, and (3) colonoscopic surveillance for relatives with Lynch syndrome. Regardless of the testing strategy used to identify probands, we assumed the same approach would be used to screen relatives and promote surveillance among those identified with Lynch syndrome.
Our model for universal testing begins with a hypothetical cohort of 150,000 newly diagnosed individuals with CRC, all of whom would be provided counseling about the potential implications of test results for themselves and family members.2 The cohort size is representative of the number of new CRC cases in the United States in 2008.8 Assuming a two thirds uptake of testing after counseling,2 100,000 patients with newly diagnosed CRC would be tested for Lynch syndrome by a program of universal voluntary testing. Our model of age-targeted testing assumes that newly diagnosed patients with CRC younger than 50 years comprise 9.2% of newly diagnosed patients with CRC,9 and testing them would detect 44% of Lynch syndrome cases.10
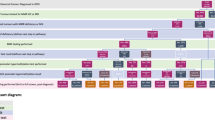
We modeled four Lynch syndrome testing strategies relative to both no testing and targeted offer of testing to newly diagnosed patients with CRC younger than 50 years (Fig. 1). The first two strategies begin with offering immunohistochemistry (IHC) testing to all newly diagnosed patients with CRC using antibodies to the MMR proteins produced by four MMR genes associated with Lynch syndrome (MSH2, MLH1, MSH6, and PMS2). An absent stain for a protein indicates a likely mutation in the associated gene. In Strategy 1, individuals with absent MLH1 protein staining are tested for the BRAF V600E mutation. If the test is positive, the individual is classified as not having Lynch syndrome. Among those with absent MSH2, MSH6, or PMS2 protein staining and those with the absence of MLH1 staining and without the BRAF mutation, sequencing/rearrangement testing of the MMR gene associated with the absent protein is conducted. Strategy 2 is similar, except without testing for the BRAF mutation.
Strategy 3 uses microsatellite instability (MSI) testing as the preliminary test. MSI testing differs from IHC because it does not predict which MMR gene is not functioning. Among those with a high MSI result, sequencing/deletion testing is performed. Strategy 4 entails offering sequencing/rearrangement testing for all four MMR genes to all patients. Henceforth, we use the term sequencing to refer to sequencing/rearrangement testing. In addition, we modeled two other strategies: IHC testing among those with MSI-positive results and BRAF mutation testing for those with IHC absent staining for MLH1 before sequencing, and direct sequencing all patients for only two MMR genes (MSH2 and MLH1) rather than all four. The results for these additional analyses are not presented here and are available from the authors upon request.
For each proband with Lynch syndrome (i.e., patient with an identified MMR gene mutation), we assumed that a specified number of adult first-degree relatives (parents, siblings, or children each of whom share roughly half the genes of the proband) would be identified, located, contacted, and offered genetic counseling relevant to Lynch syndrome. After counseling, relatives would be offered genetic testing for the known “family” mutation found in the proband. Adult relatives who agreed to genetic testing and were found to have Lynch syndrome (i.e., who carry the MMR gene mutation) would receive a recommendation for increased CRC surveillance using colonoscopy every 1–2 years starting at ages 20–25 years; we assumed 79% uptake. We incorporated into our model the estimated risks of perforation, bleeding, or death from colonoscopy and the risk of developing CRC and the risk of death from CRC derived from the EGAPP supplementary review.2 For this analysis, we assumed that a person could develop CRC not more than twice in their lifetime. Relatives found to not have Lynch syndrome were assumed to have the population risk of developing CRC and to be offered standard care (CRC screening every 10 years by colonoscopy beginning at 50 years of age).
In the baseline scenario, we assumed that an average of four first-degree adult relatives per proband would be contacted and that one half of these would agree to be counseled and tested, with two relatives tested per proband. This is a conservative assumption. In studies undertaken at the Ohio State University, an average of between five and six relatives have been tested per proband identified with Lynch syndrome.11,12 Biological first-degree relatives have a 50% chance of inheriting the family mutation. We used a 45% probability as a conservative estimate to account for the fact that some relatives tested might not be first-degree biological relatives. In the second model scenario, we assumed that testing of family members would expand beyond the first-degree relatives to include the relatives of the relatives found to have Lynch syndrome, also known as cascade screening. We assumed that cascade screening would result in a total of 12 relatives on average located per proband or approximately six relatives counseled and tested. More widespread testing could result in the inclusion of relatives with less immediate biological relationships (e.g., second-degree relatives with a 25% probability of inheriting the family mutation). Consequently, we conservatively assumed that 35% of relatives tested under this scenario would have inherited the family mutation. The two Ohio State University studies together reported that 42% of a mixture of first- and second-degree relatives who were tested carried a MMR mutation.11,12
Measuring effectiveness, costs, and cost-effectiveness
The primary measure of effectiveness used in this study was discounted life-years (LYs) saved. This is the outcome measure that has been used in most published cost-effectiveness analyses of screening for CRC in general13–16 and Lynch syndrome in particular.17–21 The number of LYs lost to CRC is calculated as the sum of the products of the number of deaths occurring within each 10-year age interval and the number of discounted LYs for each interval derived from US life table for the median age in the interval and calculated using a 3% discount rate. The number of deaths occurring within each interval is the product of the probabilities of developing CRC within a given 10-year age interval, the probability distribution of stages at diagnosis, and one minus the relative survival probabilities by stage at diagnosis.
We also calculated outcomes in terms of quality-adjusted LYs (QALYs), as is generally recommended for cost-effectiveness analyses,22 although for many life-saving interventions it makes little difference which measure is used.23,24 We adjusted future LYs by age-specific utility or quality of life weights on a scale from 0 to 1 for the US population calculated using the EQ-5D instrument.25 We assumed that the utility of survivors of nonmetastatic CRC is reduced by 0.1 for 2 years.26,27 Testing for MMR gene mutations among relatives does not seem to negatively impact quality of life.28 Cost-effectiveness ratios using QALYs are reported as a sensitivity analysis.
Based on the recommendation of the US Multi-Society Task Force on Colorectal Cancer,29 we assumed that the relatives of the probands who were found to have Lynch syndrome would undergo colonoscopy every 2 years (beginning as early as age 20 years) until 79 years of age. Colonoscopies carry a risk of complications and death, and our estimates of LYs gained were adjusted for this risk.
Following the national health care system perspective, we estimated the costs of detecting cases of Lynch syndrome, surveillance, and treatment for patients who develop cancer. The costs associated with detecting Lynch syndrome among newly diagnosed patients with CRC include the costs associated with offering testing, the costs of performing the genetic testing (both preliminary and/or sequencing), and the costs of genetic counseling both before sequencing and following the reporting of MMR gene test results. The costs of testing for Lynch syndrome among relatives include the costs of locating relatives and offering testing, the costs of genetic counseling before and after testing, and the costs of testing for the family mutation. The costs of surveillance for CRC among relatives with Lynch syndrome and the costs of treating complications associated with colonoscopies were also included. Finally, we included the treatment costs for CRC among relatives who developed CRC during their lifetime. All costs and benefits were discounted at 3%, the standard practice in US cost-effectiveness analyses based on the societal or health care system perspectives.22
We ranked strategies in terms of net costs and effectiveness (LY saved). We calculated incremental cost-effectiveness ratios (ICERs) of net costs divided by LYs in two ways. First, we calculated the ICER for each strategy relative to the next most effective strategy, as is usual in cost-effectiveness analyses after excluding strategies that are both less effective and cost more than other strategy.30 However, not all laboratories consider all testing strategies feasible. The other approach is to exclude strategies that may not be considered universally feasible. In particular, IHC testing for MMR may only be reliable when performed in specialized laboratories which participate in expert accreditation programs.7,31 Therefore, in situations where reliable IHC testing is not feasible, the ICER for Strategy 3 is calculated relative to no testing. Similarly, if the test for BRAF mutations is not available, the ICER for Strategy 2 is calculated compared with no testing.
To investigate the sensitivity of our results to our model inputs, we conducted one-way sensitivity analyses using the ranges of the parameters outlined in Table A1 in the Appendix, with the results for one strategy presented using a “tornado” diagram.
Model inputs
The model inputs for the baseline scenario, the majority of which are based on a Supplementary Evidence Review performed as part of the EGAPP pilot program,2 are presented in Table A1 in the Appendix. These include the clinical validity (sensitivity and specificity) of laboratory tests for Lynch syndrome and the proportion of Lynch syndrome attributable to each of the four MMR genes. We assumed 99.5% sensitivity and 99.96% specificity of sequencing tests. The two preliminary tests, IHC and MSI, are reported to have sensitivities of 83% and 89%, respectively, and specificities of 88.8% and 90.2%, respectively. MSI is not only more accurate than IHC but also more costly. BRAF V600E mutation testing for those with absence of MLH1 on IHC has 69% sensitivity and 99% specificity. Also included are estimates of the number of family members at risk, the number of relatives with Lynch syndrome who are expected to comply with colonoscopic surveillance every 2 years beginning at time of diagnosis (79%), the risk of adverse events related to colonoscopy, and the benefits of surveillance. We assumed that most (75%) mutation-positive relatives who did not comply with colonoscopic surveillance would undergo routine colonoscopies every 10 years beginning at age 50 years.
The age distribution of relatives with Lynch syndrome was based on expert opinion (S. Ramsey, personal communication). For the analysis considering age-targeted testing, we adjusted the age distributions of relatives to account for the fact that relatives of those <50 years would be younger than in the baseline case. To do this, we shifted the age distribution by 5 years to the left.
The distribution of CRC stages at diagnosis and the 5-year relative survival rates by stage for the general population with CRC was obtained from Surveillance, Epidemiology and End Results (SEER) data.32 It is well established that patients with MSI-positive tumors, which includes almost all CRC cases in Lynch syndrome, have markedly better survival in localized cancer than do those with MSI-negative tumors.33 On the basis of those findings, we adjusted the survival probabilities for mutation carriers diagnosed with Stages 1 and 2 CRC to 96% and 82%, respectively. In addition, surveillance colonoscopy every 2 years should be associated with earlier detection of CRC at a more localized stage and therefore a more than proportionate reduction in mortality; a recent study reported an approximately 60% lower incidence of CRC and an approximately 80% lower rate of death from cancer among mutation carriers who underwent surveillance colonoscopy.34 Consequently, we adjusted the distribution of stages among patients with Lynch syndrome who undergo biennial surveillance colonoscopy, such that 77% were assumed to be diagnosed with Stage 1 localized CRC, when compared with 40% in the SEER data.
Information on the costs of Lynch syndrome testing is difficult to obtain. For this reason, we decided to estimate each model under two different laboratory test cost scenarios (Appendix Table A2). For the baseline scenario, the economic inputs were taken mainly from the EGAPP Supplementary Evidence Review.2 These reflect 2007 Medicare reimbursement rates for genetic counseling before and after testing at the Ohio State University Comprehensive Cancer Center Clinical Cancer Genetics Program and for laboratory testing for IHC, MSI, and the four MMR genes at Mayo Medical Laboratories and Myriad Genetic Laboratories, Inc. (P. Duda and A. Smith, personal communications, respectively). For our second scenario, we obtained list prices for laboratory tests from three large, commercial laboratories (City of Hope, Mayo Medical Laboratories, and Myriad Genetic Laboratories Inc.) and calculated the median prices. These prices, which do not incorporate discounts, likely constitute an upper bound estimate of costs, thereby making testing for Lynch syndrome seem less favorable in terms of cost-effectiveness.
The costs of having a discussion with all newly diagnosed patients with CRC to offer MSI or IHC testing reflect expert opinion. The costs of locating and approaching a relative of a proband with Lynch syndrome, the costs of colonoscopy and treating possible complications resulting from colonoscopy, and the costs of treating CRC at different stages were obtained from the published literature.16–18 All costs are expressed in 2007 US dollars.
RESULTS
Outcomes and costs for each testing strategy under the baseline scenario are shown in Table 1, first for universal testing and second for age-targeted testing of patients younger than 50 years. Universal offer of testing is assumed to result in 100,000 patients tested, and the number of relatives with mutations detected is projected to range from 2197 under Strategy 1 to 2654 under Strategy 4. The expected number of discounted LYs saved per mutation carrier detected is slightly >1.0, so that LY saved range from 2346 in Strategy 1 to 2833 in Strategy 4. Strategy 4, in which all newly diagnosed individuals with CRC were offered sequencing for MMR genes, resulted in the greatest total cost, $440 million. Strategy 1 was the least expensive at $83 million. With age-targeted testing, we assumed that 9200 patients were tested resulting in 1086 to 1307 cases of Lynch syndrome detected among probands and 966 to 1162 cases detected among relatives. As expected, total program costs were lower with age-targeted testing, being 72% to 82% lower than under universal testing.
Each of the three testing strategies using a preliminary testing strategy is compared for universal testing relative to no testing for Lynch syndrome, age-targeted testing relative to no testing, and universal testing relative to age-targeted testing (Table 2). For universal relative to age-targeted testing, the ICERs range from $41,511 per LY saved for Strategy 3 (MSI testing followed by sequencing) to $22,552 per LY saved for Strategy 1 (IHC testing, followed by selective BRAF V600E mutation testing, and then sequencing). Age-targeted testing of newly diagnosed patients with CRC younger than 50 years is substantially less expensive than universal testing, $7832 per LY saved for Strategy 1 compared with no testing, one third as high as under universal testing. The cost-effectiveness ratios are equivalently lower for other testing strategies. The last column in Table 2 reports that the ICERs for universal versus age-targeted testing are $37,010 per LY saved under Strategy 1, $38,411 per LY saved under Strategy 2, assuming BRAF testing is not available, and $70,792 per LY saved under Strategy 3, assuming IHC testing is not available.
Table 2 also shows ICER calculations based on the assumption that all laboratory testing strategies are feasible. Because the strategies are similar in terms of numbers of mutation carriers detected, the ICERs for each strategy compared with the next least effective and expensive strategy are very high. The ICER for Strategy 2 is >$250,000 per LY saved for universal testing, >$60,000 per LY saved for age-targeted testing, and approximately $430,000 per LY saved for universal testing relative to age-targeted testing. Similarly, the ICERs for Strategies 3 and 4 are >$700,000 per LY saved for universal testing relative to no testing for >$1 million per LY saved for universal versus age-targeted testing. ICERs of similar magnitude are associated with alternate testing strategies in which IHC and MSI testing are conducted in sequence (results available on request). The ICER for Strategy 3 is slightly higher than for Strategy 4 under the baseline cost scenario. In decision analytic jargon, Strategy 3 is subject to “extended dominance” by a combination of Strategies 1 and 4 and should be excluded. We consider it unlikely that a laboratory might use one strategy for some specimens and a different strategy for others and therefore did not exclude Strategy 3.
Sensitivity and scenario analyses
Figure 2 shows, in descending importance, the relative influence of variables in a one-way sensitivity analysis of Strategy 1 under the baseline scenario. The most influential variables are the risk of CRC among relatives, the number of relatives per proband, increased surveillance for CRC among relatives found to have the family mutation, and the proportion of relatives accepting counseling and testing for the family mutation. Similar sensitivity analyses were conducted for each strategy and scenario, and the results are available on request. For each strategy, cost-effectiveness rises (the cost-effectiveness ratio gets smaller) as the risk of CRC among relatives, the number of relatives per proband, the number of relatives agreeing to genetic testing and to increased surveillance if found to be a carrier, and the proportion of new patients with CRC with Lynch syndrome increase. Conversely, cost-effectiveness decreases as the costs of the preliminary tests (MSI and IHC) and gene sequencing or the false-positive rates of the testing strategies increase.
Table 3 reports ICERs relative to the next most effective strategy and relative to no testing under different scenarios. Substituting the median list prices for the costs of laboratory tests, the ICER for Strategy 1 for universal testing relative to no testing is increased relative to the baseline by one third, from $22,552 to $30,331 per LY saved. Because of the relatively higher cost for BRAF mutation testing, the ICER for Strategy 2 relative to Strategy 1 is reduced from $273,915 to $170,300 per LY saved. For universal versus age-targteted testing, the ICER for Strategy 1 is almost $51,000 per LY saved and that for Strategy 2 is about $280,000 per LY saved. The ICERs for Strategies 3 and 4 are $786,030 and $1,082,378 per LY saved, respectively, under the alternate cost scenario for universal versus no testing.
If identification of relatives were to result in an average of 12 rather than four relatives identified per proband, approximately half of whom would be tested, the ICERs for the first three strategies would all be <$25,000 per LY saved for universal testing relative to no testing.
The number of QALYs gained through each strategy is 15% smaller than the number of LYs saved. This reflects the fact that most people are not in perfect health, and chronic health problems increase in frequency with age. Therefore, the average utility weight, with 1.0 representing perfect health and 0.0 representing death, is reported to decrease from 0.92 for Americans in their twenties to 0.74 for people in their eighties.25 Each LY lived is associated with less than one QALY, and the aggregate number of QALYs gained by prevention of death is 15% less than the number of LYs. Accordingly, the ICERs calculated using QALYs are 18% larger than those calculated using LYs. Thus, for example, the ICER for Strategy 1 in comparison with no testing is $22,571 per LY saved and $26,632 per QALY saved.
DISCUSSION
This study provides the first comprehensive cost-effectiveness analysis of multiple strategies to test for Lynch syndrome among all newly diagnosed patients with CRC in the United States as recommended by the EWG. Previous cost-effectiveness studies considered MSI testing as a preliminary test or modeled the use of the Bethesda or Amsterdam criteria in which testing is targeted on the basis of age and family history.17–21 We do not use Lynch syndrome-specific family history risk criteria because of the difficulty and high cost in accurately collecting and interpreting family history data for this purpose.2,3,35 Ours is the first study to model the use of IHC testing and the inclusion of BRAF V600E mutation testing for Lynch syndrome.2,36 Finally, our analysis differs from a previous US cost-effectiveness analysis that assumed that probands and relatives with Lynch syndrome would undergo prophylactic subtotal colectomy surgery, which is not common practice.18
The ICERs for universal genetic testing strategies for Lynch syndrome using a preliminary test (IHC or MSI) ranged from $12,332 to $49,272 per LY saved in comparison with no testing and from $18,778 to $85,391 per LY saved in comparison with age-targeted testing under the various cost and family testing scenarios. For the baseline assumptions on numbers of relatives tested and laboratory costs, the range of ICERs are a bit narrower, from $22,522 to $49,272 per LY saved for universal testing in comparison with no testing and from $37,010 to $70,792 per LY saved in comparison with age-targeted testing. ICERs for clinical preventive services range from negative to more than $200,000 per QALY.37,38 Screening of adults older than 50 years for CRC through colonoscopy every 10 years is reported to have an ICER of $25,000 per LY saved or less.13 Many analysts use a critical value of $50,000 or $100,000 per LY or QALY as a criterion of cost-effectiveness, although this practice has been questioned.39–41 It seems that universal testing for Lynch syndrome is well within the range of acceptable ICERs for preventive services in the United States, although probably not as low as for routine screening for CRC.
Testing strategies using IHC have the most favorable cost-effectiveness ratios, about 40% smaller than with Strategy 3 using MSI testing as a preliminary test. In particular, Strategies 1 and 2 are considerably more likely to be regarded as cost-effective using a critical value of $50,000 per LY saved than would MSI testing in the absence of IHC testing. The strategy of offering IHC testing to all newly diagnosed patients with CRC is currently recommended in Denmark7; this is the first cost-effectiveness analysis to evaluate such a universal testing strategy. Findings may differ for countries with different testing and health care costs and different family sizes. Also, the interpretation of cost-effectiveness ratios may vary across national health care systems.
The most cost-effective strategy for laboratories with the relevant expertise is the one including IHC testing, BRAF V600E mutation testing, and then targeted MMR gene sequencing. It is impossible to economically justify the application of more sensitive and costly testing strategies to all patients with CRC if a less expensive universal testing strategy is feasible. The ICERs for universal testing strategies using either preliminary MSI testing or direct MMR gene sequencing relative to the next least expensive strategy (2 or 3) exceed $750,000 per LY saved, regardless of whether the comparison is to no testing or age-targeted testing. That is, if IHC testing is feasible and reliable, it is clearly not cost-effective to use MSI testing.
Our baseline estimates, which assumed that two relatives on average would be tested for each proband with Lynch syndrome, seem conservative relative to the experience of the Ohio State University research study.9,10 Our cascade screening scenario, which assumed 12 relatives identified per proband and between five and six actually tested, yields cost-effectiveness ratios that are approximately three times as favorable as the baseline estimates. The familial hypercholesterolemia screening program in the Netherlands is reported to have tested an average of 23 relatives per proband.42
Our analysis has several limitations. First, we did not model the cost-effectiveness of universal testing relative to the use of Amsterdam or Bethesda family history-based criteria for targeted testing for Lynch syndrome. Our analysis follows the EWG2,3 in considering there to be insufficient data on the application of such criteria in routine clinical practice to model it as an alternative population-based testing strategy. Other experts might disagree. Also, we did not consider age cutoffs other than 50 years. A recent study proposed that all newly diagnosed patients with CRC younger than 60 years be tested.43
Second, our model did not adjust for relatives of probands who already know their Lynch syndrome status or already had CRC. Nationally, it is unlikely that many people already know their carrier status because there is no national screening program. In communities where programs to screen for Lynch syndrome have been in existence for some time, the situation may differ. As more people are tested for Lynch syndrome, the efficiency of testing newly diagnosed patients with CRC may decrease over time.
Third, our methodology underestimated the potential benefits if younger family members are eventually tested. The model assumes that only those who are currently aged 20 years and older are offered testing. However, adolescents who were too young to be included may be likely to be tested and start increased surveillance once they become eligible.
Fourth, we do not incorporate assumptions about ongoing compliance rates among relatives undergoing increased surveillance for CRC. If relatives who agreed to increased surveillance do not continue, the effectiveness of identification would be overstated. Studies have found that the compliance rate for increased surveillance after genetic testing for Lynch syndrome is very high,44–46 with the most recent study from Finland reporting 97% compliance after 10 years.37
Fifth, we did not model the costs or benefits of surveillance for other forms of cancer. In particular, women with Lynch syndrome are at elevated risk of gynecologic cancers. Surveillance strategies are of uncertain effectiveness, although prophylactic surgery to prevent ovarian and endometrial cancer is feasible. One analysis calculated that cost-effectiveness ratios for such strategies were approximately $200,000 per QALY gained,47 even when the costs of identifying those with Lynch syndrome are not included.
Sixth, it is important to appreciate the heterogeneity in estimates of CRC penetrance associated with MMR mutations. We assumed a cumulative CRC incidence to age 70 years among mutation carriers of 40% averaged among males and females,2 but there is a wide range of uncertainty. The true cumulative incidence could be as high as 53%48 or as low as 25%.49 If the lower estimate were assumed to be correct, the estimates of cost-effectiveness would need to be adjusted accordingly.
Seventh, there is a lack of conclusive data about the effects of MMR mutations and surveillance on staging and stage-specific survival among people who develop CRC. For example, one study reported that CRC survival was not significantly different among mutation carriers and noncarriers, although the 5-year death rate among those with localized disease was 5% among carriers and 13% among noncarriers.50 Based on similar differences in survival in localized disease among those with MSI and non-MSI tumors33 and studies among members of Lynch syndrome families, we believe that LS mutation carriers who develop localized CRC, the large majority of whom are MSI positive,51 most likely do have a survival advantage relative to noncarriers. This assumption reduces the number of deaths and LY lost to CRC among people with Lynch syndrome and proportionately reduces the projected benefits of testing strategies. Our first model, which applied the SEER estimates to mutation carriers, resulted in larger estimates of LYs saved by Lynch syndrome testing, and more favorable cost-effectiveness ratios than those that are reported here. Therefore, the revised model presented here should be regarded as a conservative projection of the potential lifesaving benefits of early detection of Lynch syndrome.
We did not model the budgetary impacts of testing for individual health care providers or payers in the fragmented US health care system. The costs of testing of new colorectal tumors for indications of Lynch syndrome would presumably be borne by probands' payers, oncology practices as part of bundled care, or both, whereas the health benefits and averted costs of care would accrue to relatives' payers. Therefore, the net budgetary impact on those paying to test newly diagnosed patients with CRC would most likely be negative. For health plans, this should balance out in the long run as they benefit from reduced costs of care for enrollees who are relatives of probands enrolled in other health plans. Consequently, the expected cost-effectiveness to an individual plan should be the same as that estimated here if all plans were to adopt the EWG recommendation.
Research is needed to assess the cost-effectiveness of testing for Lynch syndrome on an ongoing basis. It is important to track how many patients and relatives are aware of their mutation status when offered testing and how this changes over time, because greater awareness through previous testing can lessen the yield from testing. Strategies for the early detection of other forms of cancer associated with MMR gene mutations should also be considered, which has the potential to improve the projected cost-effectiveness of early detection of Lynch syndrome among relatives. Also, our assumption that face-to-face counseling would be offered before IHC testing is not necessarily valid, and use of written information materials would reduce costs and improve cost-effectiveness ratios.
CONCLUSION
We conclude that a program to offer testing for Lynch syndrome to all newly diagnosed patients with CRC is likely to be cost-effective from the perspective of the US health care system relative to other, common clinical preventive services. Using preliminary tests such as IHC or MSI results in cost-effectiveness ratios below $75,000 per LY saved compared with age-targeted testing. The most cost-effective strategy involves IHC testing first, followed by testing for the BRAF mutation among those with absent MLH1 staining, and subsequent targeted MMR gene sequencing and deletion analysis among those with absent staining for other proteins and those without the BRAF mutation, with a cost-effectiveness ratio of less than $40,000 per LY saved compared with age-targeted testing. Gene sequencing of all newly diagnosed patients with CRC for four MMR genes is not currently economically justifiable.
REFERENCES
Bonis PA, Trikalinos TA, Chung M, et al. Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications. Evid Rep Technol Assess (Full Rep) 2007; 150: 1–180.
Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN . EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med 2009; 11: 1–22.
Recommendations from the EGAPP Working Group Genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 2009; 11: 35–41.
Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 1998; 338: 1481–1487.
Pinol V, Castells A, Andreu M, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA 2005; 293: 1986–1994.
Samowitz WS, Curtin K, Lin HH, et al. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology 2001; 121: 830–838.
Vasen HF, Möslein G, Alonso A, et al. Recommendations to improve identification of hereditary and familial colorectal cancer in Europe [published online ahead of print September 18, 2009]. Fam Cancer doi:10.1007/s10689-009-9291-3.
Cancer Facts and Figures 2008. Available at: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. Accessed December 31, 2008.
US Cancer Statistics Working Group. United States cancer statistics: 1999– 2005 incidence and mortality web-based report. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2009. Available at: www.cdc.gov/uscs. Accessed February 19, 2009.
de la Chapelle A, Palomaki G, Hampel H . Identifying Lynch syndrome. Int J Cancer 2009; 125: 1492–1493.
Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005; 352: 1851–1860.
Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for lynch syndrome among patients with colorectal cancer. J Clin Oncol 2008; 26: 5783–5788.
van Gils P, van den Berg M, van Kranen H, de Wit AG . A literature review of assumptions on test characteristics and adherence in economic evaluations of colonoscopy and CT-colonography screening. Eur J Cancer 2009; 45: 1554–1559.
Pignone M, Saha S, Hoerger T, Mandelblatt J . Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2002; 137: 96–104.
Hassan C, Zullo A, Winn S, Morini S . Cost-effectiveness of capsule endoscopy in screening for colorectal cancer. Endoscopy 2008; 40: 414–421.
Sonnenberg A, Delco F, Inadomi JM . Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med 2000; 133: 573–584.
Ramsey SD, Clarke L, Etzioni R, Higashi M, Berry K, Urban N . Cost-effectiveness of microsatellite instability screening as a method for detecting hereditary nonpolyposis colorectal cancer. Ann Intern Med 2001; 135: 577–588.
Ramsey SD, Burke W, Clarke L . An economic viewpoint on alternative strategies for identifying persons with hereditary nonpolyposis colorectal cancer. Genet Med 2003; 5: 353–363.
Olsen KR, Bojesen SE, Gerdes AM, Lindorff-Larsen K, Bernstein IT . Cost-effectiveness of surveillance programs for families at high and moderate risk of hereditary non-polyposis colorectal cancer. Int J Technol Assess Health Care 2007; 23: 89–95.
Hagen A, Hessabi HK, Gorenoi V, Schonermark MP . [Cost-effectiveness evaluation of predictive molecular diagnostics using the example of hereditary non-polyposis colorectal cancer (HNPCC).]. Gesundheitswesen 2008; 70: 18–27.
Kievit W, de Bruin JH, Adang EM, et al. Cost effectiveness of a new strategy to identify HNPCC patients. Gut 2005; 54: 97–102.
Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996.
Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ . When does quality-adjusting life-years matter in cost-effectiveness analysis?. Health Econ 2004; 13: 429–436.
Tengs TO . Cost-effectiveness versus cost-utility analysis of interventions for cancer: does adjusting for health-related quality of life really matter?. Value Health 2004; 7: 70–78.
Sullivan PW, Ghushchyan V . Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006; 26: 410–420.
Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer 2000; 88: 1294–1303.
Ramsey SD, Berry K, Moinpour C, Giedzinska A, Andersen MR . Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol 2002; 97: 1228–1234.
Ramsey S, Blough D, McDermott C, et al. Will knowledge of gene-based colorectal cancer disease risk influence quality of life and screening behavior?. Public Health Genomics 2010; 13: 1–12.
Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology 2003; 124: 544–560.
Drummond M, Sculpher M . Common methodological flaws in economic evaluations. Med Care 2005; 43: 5–14.
Overbeek LI, Ligtenberg MJ, Willems RW, et al. Interpretation of immunohistochemistry for mismatch repair proteins is only reliable in a specialized setting. Am J Surg Pathol 2008; 32: 1246–1251.
Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD, National Cancer Institute. Available at: http://seer.cancer.gov/csr/1975_2005/results_merged/sect_06_colon_rectum.pdf. Accessed May 5, 2008.
Popat S, Hubner R, Houlston RS . Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005; 23: 609–618.
Stupart DA, Goldberg PA, Algar U, Ramesar R . Surveillance colonoscopy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Colorectal Dis 2009; 11: 126–130.
Ramsey SD, Burke W, Pinsky L, Clarke L, Newcomb P, Khoury MJ . Family history assessment to detect increased risk for colorectal cancer: conceptual considerations and a preliminary economic analysis. Cancer Epidemiol Biomarkers Prev 2005; 14: 2494–2500.
Bessa X, Balleste B, Andreu M, et al. A prospective, multicenter, population-based study of BRAF mutational analysis for Lynch syndrome screening. Clin Gastroenterol Hepatol 2008; 6: 206–214.
Maciosek MV, Edwards NM, Coffield AB, et al. Priorities among effective clinical preventive services: methods. Am J Prev Med 2006; 31: 90–96.
Grosse SD, Teutsch SM, Haddix AC . Lessons from cost-effectiveness research for United States public health policy. Annu Rev Public Health 2007; 28: 365–391.
Grosse SD . Assessing cost effectiveness in health care: the history of the $50,000 per QALY threshold. Exp Rev Pharmacoeconomics Outcome Res 2008; 8: 165–178.
Braithwaite RS, Meltzer DO, King JT Jr, Leslie D, Roberts MS . What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule?. Med Care 2008; 46: 349–356.
Weinstein MC . How much are Americans willing to pay for a quality-adjusted life year?. Med Care 2008; 46: 343–345.
Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, Scheerder RL, Kastelein JJ . Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001; 357: 165–168.
Schofield L, Watson N, Grieu F, et al. Population-based detection of Lynch syndrome in young colorectal cancer patients using microsatellite instability as the initial test. Int J Cancer 2009; 124: 1097–1102.
Wagner A, van Kessel I, Kriege MG, et al. Long term follow-up of HNPCC gene mutation carriers: compliance with screening and satisfaction with counseling and screening procedures. Fam Cancer 2005; 4: 295–300.
Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP . Ten years after mutation testing for Lynch Syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol 2009; 27: 4793–4797.
Halbert CH, Lynch H, Lynch J, et al. Colon cancer screening practices following genetic testing for hereditary nonpolyposis colon cancer (HNPCC) mutations. Arch Intern Med 2004; 164: 1881–1887.
Kwon JS, Sun CC, Peterson SK, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer 2008; 113: 326–335.
Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology 2009; 137: 1621–1627.
Quehenberger F, Vasen HF, van Houwelingen HC . Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet 2005; 42: 491–496.
Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med 2006; 354: 2751–2763.
Watson P Lin KM Rodriguez-Bigas MA et al. Colorectal carcinoma survival among hereditary nonpolyposis colorectal carcinoma family members. Cancer 1998; 83: 259–266.
Acknowledgements
The authors thank Rena Conti, James Gudgeon, Ira Lubin, Wolf Rogowski, Susan Snyder, David Veenstra, and members of the EGAPP Working Group (see www.egappreviews.com for names/affiliations) for their helpful comments on prior versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: Drs. Mvundura, Grosse, and Palomaki declare no conflict of interest. Dr. Palomaki is partially supported as a consultant to the EGAPP Initiative, funded by the Centers for Disease Control and Prevention through a contract with McKing Consulting Corporation. Ms. Hampel has two potential conflicts to disclose. She has received honoraria From Myriad Genetic Laboratories, Inc. for Advisory Group work and from Falco Biosystems for giving a lecture.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Rights and permissions
About this article
Cite this article
Mvundura, M., Grosse, S., Hampel, H. et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med 12, 93–104 (2010). https://doi.org/10.1097/GIM.0b013e3181cd666c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e3181cd666c
Keywords
This article is cited by
-
Impact of free cancer predisposition cascade genetic testing on uptake in Singapore
npj Genomic Medicine (2019)
-
Effective Identification of Lynch Syndrome in Gastroenterology Practice
Current Treatment Options in Gastroenterology (2019)
-
Universal tumor screening for Lynch syndrome: perspectives of Canadian pathologists and genetic counselors
Journal of Community Genetics (2019)
-
Assessing colorectal cancer mismatch repair status in the modern era: a survey of current practices and re-evaluation of the role of microsatellite instability testing
Modern Pathology (2018)
-
A tailored approach to BRAF and MLH1 methylation testing in a universal screening program for Lynch syndrome
Modern Pathology (2017)