Abstract
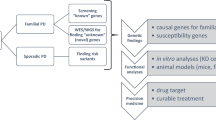
During the past decade five genes have been identified that are important in autosomal dominant and autosomal recessive forms of Parkinson disease. The identification of these genes has increased our understanding of the likely pathogenic mechanisms resulting in disease. However, mutations in these genes likely contribute to disease in fewer than 5% of all cases of Parkinson disease. Thus, researchers have continued to search for genes that may influence disease susceptibility. Molecular diagnostic testing is currently available for four of the genes mutated in Parkinson disease. Evidence for reduced penetrance, possible effects of haploinsufficiency, and the identification of nondisease causing polymorphisms within several of these genes has made genetic counseling challenging. Current recommendations are to limit molecular testing only to those individuals who are symptomatic. Furthermore, because treatment is unaltered by the presence or absence of mutations in these genes, restraint is recommended when considering the value of screening for mutations in a clinical setting.
Similar content being viewed by others
Main
Parkinsonism is a broad term referring to all clinical states characterized by tremor, slowed movement (bradykinesia), rigidity, and postural instability. Parkinson disease (PD) is the primary and most common form of parkinsonism, and is the second most common neurodegenerative disorder after Alzheimer disease (AD). It affects more than 1% of 55-year-old individuals and more than 3% of those over 75 years of age.1 The age of disease onset is widely variable, ranging from juvenile to very late in life with an average age of onset of 60 years. Generally, individuals with onset before age 20 are considered to have juvenile-onset; those with onset between 20 and 50 years of age are classified as having early-onset; and those with onset after age 50 are referred to as late-onset.
The overall age- and gender-adjusted incidence rate is 13.4 per 100,000. There is a higher prevalence among men (19.0 per 100,000) than among women (9.9 per 100,000).2 PD seems to have a similar incidence across most ethnicities; however, it may be less common among African Americans.2
Psychiatric manifestations can be a prominent feature of disease and may include depression and visual hallucinations. Depression occurs in 25–50% of PD patients.3–5 Later in disease progression, dementia eventually occurs in 20–40% of cases.6
A number of risk factors have been evaluated for their role in disease susceptibility. Smoking has consistently been reported to result in a 50% decrease in the risk of disease.7–11 The effect of smoking is dose-dependent and temporal, with the protective effect of smoking greater for those who quit later in life.8,10,11 Caffeine has a similar well-documented, dose-dependent protective effect on PD.8,12–14 The protective effect of caffeine in women seems to be modulated by the use of postmenopausal hormones, with protection only being conferred in women that did not undergo estrogen replacement therapy.15,16 Serious head trauma has also been found in multiple studies to increase the risk of PD.17,18 Other factors that have been inconsistently reported to modulate disease risk include well water,19,20 pesticide use,21–23 and rural living.19,24 The results of 29 studies are summarized in Lai et al.25
In the mid 1980s, a contaminate found in a synthetic form of heroin was found to cause rapid-onset, levodopa-responsive parkinsonism.26 The responsible toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), is metabolized and then preferentially enters dopaminergic neurons at terminal reuptake sites. Toxicity results from the inhibition of complex I of the electron transport chain, thus implicating mitochondrial dysfunction as a notable cause of PD. Subsequently, it was shown that complex I activity is selectively diminished in the substantia nigra of patients with idiopathic PD as well.27 The identification of an environmental agent that can induce parkinsonism also provided a means of creating animal models of PD. Various doses and protocols have been able to mimic acute and chronic forms of neurodegeneration by eliciting neurochemical changes such as decreased levels of dopamine, increased oxidative damage, decreased concentrations of antioxidants and aggregates containing alpha-synuclein, a principal component of Lewy bodies.28 Another important contribution from the study of MPTP is the reminder that PD has a very complex etiology. Although over 400 individuals were thought to have been exposed to MPTP, only a small fraction developed this severe form of parkinsonism,29 thus illustrating the multifactorial nature of disease risk.
Studies from around the world have provided evidence that genetic risk factors are involved in the pathogenesis of the idiopathic form of PD. Estimates of the relative risk to first-degree relatives of an affected individual range from 2.7 to 3.5 in the United States,30,31 2.9 in Finland,32 6.7 in Iceland,33 7.7 in France,34 3.2 in three centers within Europe,35 5.0 in Canada,36 13.4 in Italy,37 and 7.1 in Germany.38
DIAGNOSTIC FEATURES OF PD
The cardinal pathologic feature of PD is the loss of dopaminergic neurons in the substantia nigra with intracytoplasmic inclusions (Lewy bodies) in the remaining, intact nigral neurons.39,40 Symptoms of PD typically onset when 50–80% of the dopaminergic neurons in the substantia nigra are no longer functioning. Traditionally, the presence of Lewy bodies was required for pathologic confirmation of PD; however, it has been recognized recently that nigral pathology may occur in the absence of Lewy bodies.
Because a diagnosis of PD can only be made through documentation of salient clinical features and postmortem verification of Lewy bodies, some diagnostic uncertainty is unavoidable. The careful application of diagnostic criteria derived from existing clinicopathologic studies can increase the positive predictive value of diagnosis to over 95%.41 Presence of resting tremor, response to dopamine agents, asymmetric onset of symptoms, and the absence of atypical features that suggest other diagnoses are all criteria that can be used to increase the certainty of diagnosis. Functional imaging techniques such as positron emission tomography or single photon computed emission tomography using radioactively labeled ligands of the presynaptic dopaminergic neurons can support the diagnosis but are usually limited to a research setting. By maximizing the specificity of the criteria, the sensitivity of the criteria falls dramatically, thereby excluding as many as one third of true cases.42 Although these diagnostic criteria are ideal for a genetic research study, they may not be useful for making a clinical diagnosis.
Several other neurologic entities must be considered as part of the differential diagnosis of PD. They include parkinsonism-predominant multiple system atrophy (formerly called striatonigral degeneration), progressive supranuclear palsy, corticobasal degeneration, essential tremor, drug-induced parkinsonism, postencephalitic conditions, Lewy body dementia, and AD. Parkinsonism can also be a prominent feature of some Mendelian disorders, including spinocerebellar ataxias (i.e., MJD/SCA3 and SCA2), Huntington disease, dopa-responsive dystonia, familial prion disease, frontotemporal dementia with parkinsonism-17, Wilson disease, and X-linked dystonia-parkinsonism syndrome (Lubag). Laboratory or radiologic studies cannot be used to confirm PD; however, they are useful in excluding alternative diagnoses, such as stroke, tumor, and thyroid disease.
GENETICS OF PD
For many years PD was thought to be strictly influenced by environmental factors without a substantial genetic contribution to disease etiology. However, research has since demonstrated the importance of genetic factors in at least a subset of PD patients (Table 1). Linkage analyses in both autosomal dominant and autosomal recessive kindreds have identified the genes resulting in Mendelian (single gene) forms of parkinsonism. Mutations in SNCA (PARK1) and LRRK2 (PARK8) result in autosomal dominant PD. Linkage to a third locus (PARK3) has been reported, but the gene has not yet been identified. A fourth locus, UCHL1 (PARK5) has been implicated, but not confirmed. Mutations in three known genes, PRKN (PARK2), DJ-1 (PARK7), and PINK1 (PARK6), result in autosomal recessive PD. The role of these genes and the clinical characteristics of patients with mutations are described in more detail below. Importantly, the mutations identified to date in these genes likely cause PD in fewer than 5% of patients.
Our understanding of the genetic contribution to the risk of PD is likely still incomplete, despite the identification of several PD causative genes. Therefore, analyses are ongoing that seek to identify genetic variants that increase or decrease the risk of PD. Typically, these analyses have focused on genes that are not causative but rather are susceptibility loci. Several susceptibility genes have also been implicated; although their role in most cases is not yet conclusively replicated.
Autosomal dominant PD
PARK1: SNCA
The first mutation identified in PD subjects was found in an autosomal dominant pedigree of Italian descent, called the Contursi kindred.43 Members of this family have a mutation in the gene (SNCA; A53T in exon 4) that codes for the protein alpha-synuclein. The affected members of the family have similar clinical and pathologic findings to those with idiopathic PD, including a response to levodopa and the presence of Lewy bodies. However, the mean age of onset of affected individuals is 46 years. The same mutation (A53T in exon 4) was also found in nine Greek families44; given the close historical ties between Greece and Southern Italy, it has been suggested that the mutation may be the result of a common founder effect.45
Additional point mutations in SNCA have been reported. A German family was found to have an A30P in exon 3, which resulted in disease.46 A Spanish kindred with autosomal dominant parkinsonism and Lewy body dementia was found to have an E46K mutation.47 Subsequent sequence analyses in thousands of patients have shown that point mutations in SNCA are an uncommon cause of familial or simplex PD.48–54
Further analyses have shown that other types of alterations in SNCA can result in PD. A triplication of a large chromosomal region containing SNCA has been shown to cause autosomal dominant PD.55 Duplication of SNCA has also been found to cause disease.56–58 Analyses of these families suggest that gene triplication results in an earlier onset of disease as compared with gene duplication. Extensive molecular analyses initially indicated that gene multiplication is a rare mechanism for PD causation.59–61 However, a recent study found three SNCA duplications in a sample of 906 screened subjects (<1%).62 Only one of the three positive patients had a positive family history of PD. Importantly, in each of these three families, asymptomatic members were found who carried the duplication, suggesting that penetrance of a SNCA duplication may be incomplete.
Although mutations in SNCA have been known to cause PD for nearly a decade, the mechanism by which these mutations lead to disease is poorly understood. It is thought that aberrant aggregation of the protein results in cell damage and ultimately neuronal death. However, much more research is required to understand how mutations in SNCA or multiplication of SNCA result in parkinsonism.
Variation in the promoter region of SNCA has been reported to increase susceptibility for PD,63 and a meta-analysis of 2692 cases and 2652 controls has further bolstered evidence that this marker, termed Rep1, is associated with a slight, but significant, increase in the risk of PD.64 Rep1 is a dinucleotide repeat sequence that has three prominent allele sizes. Analysis of the surrounding DNA suggests that two domains flanking the Rep1 repeat interact to enhance expression of SNCA whereas the repeat acts as a negative modulator.65 In addition, different alleles can vary the expression levels of SNCA in SH-SY5Y cells by upto threefold.65 It is possible that even a subtle increase in expression could, over the course of many decades, predispose an individual to develop PD.
PARK8: LRRK2
Mutations in the most recently identified gene, LRRK2, have been found among patients with a later age of onset and seem to result in typical, idiopathic PD.66,67 Nearly a dozen different mutations have been reported; the most common, G2019S, has been found in approximately 5–7% of familial, autosomal dominant PD68–70 and 1–2% of sporadic cases.71 These estimates have been derived from mostly North American and Northern European populations; the G2019S mutation seems to be extremely rare in East Asia.72,73 Nevertheless, the G2019S mutation is the most common single cause of PD identified to date.
The age of onset for individuals with the G2019S mutation is highly variable (from age 35 to 78 years). Studies in autosomal dominant pedigrees segregating the G2019S mutation estimated the penetrance of the mutation to be relatively high. In one study, the penetrance at age 50 was 17% but rose to 85% by age 70 years.70 A second study found the penetrance to be 33% at age 55 and 100% by age 75.74 However, it is quite likely that the estimated penetrance of the G2019S mutation is greater in pedigrees with autosomal dominant inheritance as compared with estimates from pedigrees without such a strong family history of disease. Therefore, to avoid an ascertainment bias, a recent study ascertained LRRK2 mutation carriers through the molecular screening of a large series of consecutive PD patients who were tested regardless of their family history of PD.75 Testing of the relatives of the PD patients found to have the G2019S mutation yielded much lower estimates of disease penetrance. In this sample, the penetrance by age 50 was 17%, and at age 70 was 54%. In this study, five carriers of the G2019S mutation were over the age of 75 (up to age 89) and did not have signs of disease. The relatively low penetrance rates raise substantial concerns with regard to molecular testing, as discussed later.
The frequency of the G2019S mutation has been reported to be substantially higher among Ashkenazi Jews76 and North African Arabs,77,78 and to a lesser extent in Portugal.79,80 Haplotype studies suggest a founder effect, which may explain the lower to null frequencies of this mutation in populations further from these regions. Homozygotes and heterozygotes for the G2019S mutation have similar clinical features and both genotypes demonstrate reduced penetrance.81
Imaging studies of patients with G2019S, R1441C, and Y1699C LRRK2 mutations have been indistinguishable from those obtained from patients with idiopathic PD not known to carry LRRK2 mutations.82 The vast majority of LRRK2 cases that have come to autopsy have been found to have brainstem or transition Lewy body disease, typical of idiopathic PD.83 However, some LRRK2 mutation-positive cases have been reported with disparate neuropathology, which has included Lewy bodies restricted to the brainstem, diffuse Lewy bodies, neurofibrillary tangles and abnormal tau deposits, and frontotemporal lobar degeneration with ubiquitinated neuronal intranuclear inclusions.66,82,84,85
LRRK2 codes for a protein kinase that contains five functional domains in the C-terminal half of the protein.86 Allelic heterogeneity has been observed in this gene with disease-producing mutations identified in all five domains. Despite its large size, exhaustive screening of all 51 exons has been performed by several studies and additional mutations have been reported.87–91 However, each of these novel mutations has proven to be quite rare and was found in only one or a few families.92,93 Several LRRK2 substitutions have been reported (i.e., R1514Q) that have been found at similar frequency in PD cases and controls and therefore are unlikely to be disease-producing.94
Two studies have corroborated that LRRK2 is primarily found in the cytosol, but a small proportion of the protein associates with the outer membrane of the mitochondria.95–97 It also seems that mutations in LRRK2 do not affect the protein's steady-state levels, localization, or turnover. Instead, the mutations studied thus far at the molecular level all seem to up-regulate kinase activity and increase autophosphorylation.95,96
Other autosomal dominant loci
The PARK3 locus was originally identified in several families of German descent who were segregating an autosomal dominant form of disease.98 Clinical symptoms in these families are similar to those in typical PD, with a mean age of onset of 59 years and Lewy body pathology. In 1998, a linkage study mapped a putative gene to Chromosome 2p13 and the locus was termed PARK3.98 The causative mutation has not been definitively identified; however, there is some evidence that it is influenced by variation in or around SPR, which codes for an enzyme called sepiapterin reductase that is implicated in dopamine biosynthesis.99,100 No pathogenic mutations in sepiapterin reductase (SPR) gene have been identified in families with PD101; however, a mutation was identified in an individual with dopa-responsive dystonia.102
Analyses in a single sibling pair of German heritage reported the cosegregation of disease with an I93M mutation in UCHL1 (PARK5). The clinical features in these siblings were similar to those seen in idiopathic PD and included a response to levodopa and age of onset at 49 and 50 years.103 Molecular testing of hundreds of individuals with PD has not identified any others carrying the I93M or any other mutations in UCHL1; thus, the initial report103 may be the result of a coincidental polymorphism.104,105
Autosomal recessive PD
PARK2: PRKN
Mutations in the PRKN gene (PARK2), located on Chromosome 6, were initially reported in a sample of Japanese families with autosomal recessive, juvenile parkinsonism.106 Subsequent molecular screening has identified the majority of the mutations in subjects with onset up to age 40. Patients with PRKN mutations have typical PD features, often with lower-limb dystonia. Disease progression is slow. Sustained response to levodopa is observed as well as early, often severe, dopa-induced complications (fluctuations and dyskinesias).107 Interestingly, there have been reports that patients with PRKN mutations in some instances lack the characteristic Lewy bodies found in most cases of idiopathic PD.108,109
As a result of extensive molecular testing, over 100 mutations have been reported throughout PRKN.110 Mutations have included point mutations as well as exon rearrangements, including both deletions and duplications.106,111–118 Several studies have sought to determine the origins of certain mutations by analyzing their surrounding haplotypes110,119 In general, whole exon rearrangements are thought to represent independent events whereas certain missense mutations may be the result of a founder effect. Mutations have been found in each of the 12 exons of PRKN.
Parkin is an E3-type ubiquitin protein ligase that is involved in the degradation of specific proteins, including alpha-synuclein, a primary component of Lewy bodies. Mutations in PRKN are thought to disrupt this important E3 activity and result in a loss of normal protein function. Although all parkin substrates have yet to be definitively identified, it is hypothesized that the accumulation of these proteins, which were to have been ubiquitinated, results in selective cell death of neurons in the substantia nigra and locus coeruleus.120
Several studies have reported that a PRKN mutation in only one of the two copies of the PRKN gene may increase susceptibility for PD or may even result in an autosomal dominant pattern of inheritance.109,118,121,122 However, a recent study123 has found similar rates of heterozygous missense PRKN mutations in controls and PD subjects. Although this study did not examine the rates of dosage mutations in cases and controls, it was the first to fully sequence a large number of controls, suggesting that the higher rates of heterozygous PRKN mutations previously observed in cases may have been due to bias. These results would seem to reduce the likelihood that a single mutation in PRKN may increase the risk for PD. Further data are clearly warranted to address these disparate results and also to provide more accurate estimates of the penetrance of PRKN mutations as well as genotype/phenotype correlations.
PARK6: PINK1
Mutations in PINK1 were initially identified in early onset, autosomal recessive kindreds. Point mutations, frameshift mutations, and truncating mutations have been reported throughout the gene. PINK1 mutations account for 1–7% of early onset or autosomal recessive PD in white patients.124–126 The frequency seems to be higher in Japanese patients with estimates that nearly 9% of autosomal recessive PD patients have a mutation in this gene.127 Patients with PINK1 mutations seem to have clinical features that resemble late onset PD; however, they may have atypical features such as dystonia at onset, sleep benefit, and psychiatric disturbances.124,128–130 Penetrance of disease mutations seems to be high.127,128,131 Wild-type PINK1 encodes a protein that localizes to the mitochondria124 and has been hypothesized to have a neuroprotective role against mitochondrial dysfunction and proteasomally induced apoptosis. It is hypothesized that mutations in PINK1 may result in increased susceptibility to reactive oxygen species and other cellular stressors and thereby may result in PD.132 Similar to other forms of autosomal recessive forms of PD, there is debate as to whether a PINK1 mutation in the heterozygous state may increase the risk for PD.133–136
PARK7: DJ-1
Only seven families have been identified worldwide with autosomal recessive, early onset PD due to mutations in DJ-1. The reported mutations consist of missense mutations, whole exon deletions, a frameshift mutation, and a splice site mutation found in either a homozygous or compound heterozygous state.137–142 Through extensive molecular screening, it is estimated that mutations in DJ-1 account for <1% of all cases of early onset PD.136,141,143–146
DJ-1 encodes a ubiquitous, highly conserved protein that plays a role in oxidative stress.147,148 In particular, DJ-1 seems to act as an intracellular sensor for such damage when the cysteine residue at position 106 becomes oxidized.149,150 Once oxidized, it is thought that DJ-1 acts as a chaperone for proteins such as alpha-synuclein, thereby preventing alpha-synuclein fibrillation, protein aggregation, and misfolding.151,152DJ-1 protein levels are elevated in the cerebrospinal fluid of individuals with idiopathic PD, particularly for those in the earlier stages of disease, suggesting that DJ-1 might be useful as a biomarker for neurodegenerative disease.153DJ-1 knockout mice have nigrostriatal dopaminergic deficits and are hypersensitive to the effects of the neurotoxin MPTP.154
Susceptibility genes for PD
Although the study of large families segregating Mendelian forms of PD have provided substantial insight regarding the etiology and pathogenesis of PD, mutations in these genes have been found in fewer than 5% of all PD patients. Previously published data suggest that the risk of PD among the first-degree relatives of an affected individual is 2 to 14 times higher than the risk in the general population. These data would suggest that additional loci contribute to the risk of PD.
Several different approaches have been used to identify these additional loci. Several research groups have identified multiplex PD families, typically those with at least a sibling pair with disease, and have performed a whole genome linkage screening to identify chromosomal regions linked to the risk of PD or the age of PD onset.155–161 Chromosomes 5 and X have appeared in multiple linkage studies. Analyses combining data from two studies was not able to replicate linkage to Chromosome 5.162 Additional analyses on the X chromosome have identified several candidate genes; however, none have been verified.
Another approach that has been used to identify genes contributing to PD susceptibility has been to compare allele and genotype frequencies in a sample of PD cases and neurologically normal controls. This approach has been used in analyses of the entire genome as well as others that were limited to the study of a single candidate gene. Two genome-wide association studies have been performed.163,164 Unfortunately, there has been little overlap between the two studies and a few independent studies have been published that have not confirmed the initially associated regions or single-nucleotide polymorphisms.165–168 Although discouraging, the design of the two initial genome-wide association studies were quite different in the types of PD cases and controls selected, and these factors may account for the discrepant results. In addition, there is evidence that the sample size necessary to detect and replicate susceptibility alleles with small effect sizes may require a sample that is 10 times that of those used in these first two studies.
The evaluation of particular candidate genes has led to the identification of several susceptibility genes; however, most have failed to consistently replicate in other populations.169 An exhaustive review of all genes analyzed as a candidate gene for PD is beyond the scope of this review. Rather, we have summarized the genes that have been the subject of the most intense focus in recent years.
NR4A2
NR4A2, also known as Nurr1, encodes a member of a nuclear receptor superfamily that is essential for the differentiation of the nigral dopaminergic neurons.170–172 Mice in which both alleles of the NR4A2 gene have been inactivated lack mesencephalic dopaminergic neurons.173,174 Mice in which only one copy of the NR4A2 gene is inactivated demonstrate greater susceptibility to nigral injury and have features consistent with PD.175 A polymorphism in exon 6 of NR4A2 was found to be more frequent in familial cases of PD. A year later, two different mutations in exon 1 were found to segregate with PD in 10 families.176 The age at onset of disease and clinical features of these 10 probands did not differ from those of individuals with typical PD. Le et al.176 also presented data suggesting that dopaminergic dysfunction can result from mutations in NR4A2. However, neither the association with the polymorphism nor either mutation has been found in other large studies of familial and sporadic PD.177–183
SNCAIP
Similar to alpha-synuclein (the protein encoded by SNCA), synphilin-1, the protein encoded by SNCAIP, is a substrate of parkin (PRKN). It has been shown to interact directly with alpha-synuclein and is found, along with parkin and alpha-synuclein, in Lewy bodies. The same mutation (R621C) was reported in two individuals with late-onset idiopathic PD (age of onset: 63 and 69 years) who had no apparent family history of disease.184 In a group of 328 German individuals with familial or sporadic PD, the R621C mutation was the only genetic variant found and was not seen in 351 control individuals. The two mutation carriers share the same rare alleles for five of the six microsatellite markers genotyped in the chromosomal region around the SNCAIP gene, suggesting that this variant was inherited from a common ancestor.184
Functional studies of synphilin-1 indicate that abnormal protein can form cytoplasmic inclusions in transfected cells and that cells transfected with the R621C polymorphism were more susceptible to apoptosis than cells expressing wild-type synphilin-1. The role of synphilin-1 in PD susceptibility has not been replicated185–187; therefore, although it may be important in disease pathogenesis, it is likely to be a rare cause of disease.
APOE
Variation in apolipoprotein E (APOE) has been strongly associated with the risk of AD. Variation in two amino acid residues results in three observed alleles (ε2, ε3, and ε4). The ε4 allele is found in roughly a third of whites and is associated with a 2-fold risk of disease when found in the heterozygous state and a 5–10-fold increase in risk when homozygous.188 Dementia is estimated to occur in 30% of patients with PD,6 and a large longitudinal study of AD patients found that at least one motor sign of PD was detected in 44% of patients during at least one of their study visits.189 Because of this overlap, several studies have evaluated whether APOE genotype is a risk factor for PD.
Evidence for the role of APOE in PD has been inconsistent.190,191 Several recent studies have found the ε4 allele to be associated with PD192–194; however, an earlier meta-analysis found a marginal association only with the ε2 allele.195 A more consistent association of the ε4 allele has been with an increased risk of dementia within a PD sample.190,196,197 Several larger genetic studies have also found the ε4 allele to be significantly associated with an earlier age of PD onset.191,197–199
GBA
Individuals with mutations in both copies of GBA, the gene encoding glucocerebrosidase, have Gaucher disease. This disease is found at a high rate among Ashkenazi Jews. A single mutation in GBA has been reported to confer an increased risk of PD (odds ratio [OR]: 7.0; G-test P < 1 × 10−10), particularly in populations of Ashkenazi Jewish descent.200
All studies that have sought to confirm this association have found an increased frequency of GBA mutations in cases versus controls; however, a few studies failed to find a difference that was statistically significant, possibly due to lack of power (β < 0.20).201 Studies of a Canadian cohort (OR: 7.3; P = 0.03),202 two US cohorts (OR: 5.6; P = 0.01),203,204 a Venezuelan cohort (OR: 4.1; P = 0.17),205 a Norwegian cohort (OR: 1.3; P = 0.56),206 and a Chinese cohort (OR: 4.1; P = 0.16)201 have all found heterozygous GBA mutations at higher rates among cases.
MAPT
The encoded protein, more frequently referred to as tau, is abundantly expressed in neurons and plays an important role in organizing and maintaining cell structure.207 Aggregation of tau is a pathologic hallmark of several neurodegenerative disorders, collectively known as tauopathies. These include Pick disease and AD, as well as several disorders with parkinsonian features: progressive supranuclear palsy, corticobasal degeneration, and frontotemporal dementia with parkinsonism. Even some individuals that present as typical PD can have tau pathology, as was discussed earlier regarding individuals with LRRK2 mutations. A variety of mutations have been identified in the MAPT gene, primarily in patients with frontotemporal dementia; however, clinical and phenotypic heterogeneity, even within the same family, argues that the tau protein's role in the brain is complex. Together with additional genetic or environmental factors, tau dysfunction can have an effect on many neuronal processes.207 Recent evidence demonstrates that one of those factors that tau interacts with is alpha-synuclein208 and that one of tau's neuronal perturbations may lead to PD.
Two meta-analyses performed using several thousand cases and controls suggest that a large haplotype block containing the MAPT gene is associated with a small but significant increase in risk for PD.209,210 This was recently confirmed by another large study of 1762 PD patients and 2010 control subjects and was found to be consistent across age, gender, and family history.211 The deleterious haplotype (H1) and the protective haplotype (H2) actually represent groups of subhaplotypes that have formed independently; however, associations with any of these subhaplotypes have so far been inconsistent.211 The single-nucleotide polymorphisms that define the parent haplotypes of H1 and H2 are in complete linkage disequilibrium with each other (r2 = 1), indicating that the functional variation could be anywhere within this large 900-kb region and not necessarily within any segment of MAPT. Complex permutations of alternative splicing lead to many different isoforms of tau; so if the association with H1 is due to variation that were to upset this delicate balance of isoforms, it may help to explain the variety of different neurodegenerative phenotypes that exhibit tau pathology.
Mitochondrial DNA
Mitochondrial dysfunction, particularly with regard to complex I of the electron transport chain, has been implicated in the pathogenesis of PD. Variations within mitochondrial DNA have been thought to influence susceptibility to neurodegeneration for many years212,213; however, exhaustive sequencing of the mitochondrial genome has not yet revealed mutations that consistently associate with PD.214,215 Population-based studies have also been undertaken to identify mitochondrial haplotype groups that increase the risk of PD216–218; however, these too have provided conflicting results. More recently, two groups have shown that somatic deletions of mitochondrial DNA are found at a higher rate within substantia nigra neurons from individuals with PD as well as the elderly in general.219,220
MOLECULAR DIAGNOSTIC TESTING IN PD
Molecular diagnostic testing is currently available for PRKN (PARK2), PINK1 (PARK6), DJ-1 (PARK7), and LRRK2 (PARK8). However, molecular testing and genetic counseling is challenging in PD patients and their families because of the varied patterns of inheritance that has been observed. Clear review of the family history and clinical symptoms is essential in prioritizing the molecular tests. Importantly, clinical recommendations are not altered based on the presence or absence of a particular molecular mutation. Therefore, molecular diagnostic testing is not essential in the current management of PD patients.
For PD patients with an early onset of disease, it has been estimated that 50% of those with onset before age 40 have a mutation in PRKN, PINK1, or DJ-1. However, given the rarity of PINK1 or DJ-1 mutations, initial testing of PRKN would seem to be the most cost effective strategy. Based on the distribution of age of onset of patients with PRKN mutations, it has been recommended that those patients with onset prior to age 40 be considered for screening for PRKN mutations. Although mutations have been found in those who onset above age 40, the rate is quite low and therefore testing is not likely to be cost effective.
The significance of a mutation in only one of the two PRKN alleles is not yet known. Therefore, it is strongly recommended that PRKN testing be undertaken only in those who have already onset with disease; molecular screening of PRKN is not recommended as a test for presymptomatic individuals. Another important caveat in PRKN testing is the presence of sequence variants that seem not to be disease-producing. A number of such variants have been reported in PRKN and several are at relatively high frequency. Therefore, careful attention to the type of sequence variation reported in molecular testing is essential.
In patients with early onset PD who have been tested for PRKN and found to be negative, it may be appropriate to consider screening PINK1 and DJ-1. However, mutations in both of these genes are much less frequent than those in PRKN.
For patients with typical, later onset idiopathic PD, screening for the three most common mutations in LRRK2 may be considered. Based on previous studies, it is clear that this mutation is much more likely in those PD patients with a family history consistent with autosomal dominant PD. Typically these patients will report affected parents or siblings. Of note, given the size of the LRRK2 gene and rarity of the other reported mutations, diagnostic molecular testing is only available for mutations found at codons 1441, 2019, and 2020. As a result, a false-negative result is possible.
Similar to the caveats with the testing of autosomal recessive genes, it is strongly recommended that LRRK2 testing be performed only for those patients with a diagnosis of PD. Studies have clearly demonstrated that the G2019S mutation is not fully penetrant, even when homozygous. Therefore, to avoid providing a molecular test result without accurate predictive data, it is strongly recommended that the G2019S test not be used as a presymptomatic test for PD.
SUMMARY
Over the past decade, substantial progress has been made in our understanding of the genetic contribution to PD susceptibility. Five genes have been identified that cause either autosomal dominant or autosomal recessive PD. Molecular diagnostic testing is available for the genes contributing to the most common type of autosomal recessive (PRKN) and autosomal dominant (LRRK2) PD. In addition, molecular screening is also available on a clinical basis for two genes that cause relatively rare forms of autosomal recessive PD (PINK1, DJ-1). In all cases, molecular testing is only recommended for individuals with diagnosed PD. For both PRKN and LRRK2, all mutations are not equally penetrant, and accurate penetrance estimates are not yet available. Therefore, it is strongly recommended that molecular diagnostic testing not be performed for presymptomatic individuals.
References
de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, et al. Prevalence of parkinsonism and Parkinson's disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson's disease. J Neurol Neurosurg Psychiatry 1997; 62: 10–15.
Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 2003; 157: 1015–1022.
Dooneief G, Mirabello E, Bell K, Marder K, et al. An estimate of the incidence of depression in idiopathic Parkinson's disease. Arch Neurol 1992; 49: 305–307.
Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, et al. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 1992; 55: 377–382.
Tandberg E, Larsen JP, Aarsland D, Cummings JL . The occurrence of depression in Parkinson's disease. A community-based study. Arch Neurol 1996; 53: 175–179.
Emre M . Dementia associated with Parkinson's disease. Lancet Neurol 2003; 2: 229–237.
Checkoway H, Powers K, Smith-Weller T, Franklin GM, et al. Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol 2002; 155: 732–738.
Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ . A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 2002; 52: 276–284.
Allam MF, Campbell MJ, Hofman A, Del Castillo AS, et al. Smoking and Parkinson's disease: systematic review of prospective studies. Mov Disord 2004; 19: 614–621.
Thacker EL, O'Reilly EJ, Weisskopf MG, Chen H, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology 2007; 68: 764–768.
Ritz B, Ascherio A, Checkoway H, Marder KS, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol 2007; 64: 990–997.
Ross GW, Abbott RD, Petrovitch H, Morens DM, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 2000; 283: 2674–2679.
Ascherio A, Zhang SM, Hernan MA, Kawachi I, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol 2001; 50: 56–63.
Tan EK, Tan C, Fook-Chong SM, Lum SY, et al. Dose-dependent protective effect of coffee, tea, and smoking in Parkinson's disease: a study in ethnic Chinese. J Neurol Sci 2003; 216: 163–167.
Ascherio A, Chen H, Schwarzschild MA, Zhang SM, et al. Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology 2003; 60: 790–795.
Ascherio A, Weisskopf MG, O'Reilly EJ, McCullough ML, et al. Coffee consumption, gender, and Parkinson's disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. Am J Epidemiol 2004; 160: 977–984.
Factor SA, Weiner WJ . Prior history of head trauma in Parkinson's disease. Mov Disord 1991; 6: 225–229.
Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, et al. Head trauma preceding PD: a case-control study. Neurology 2003; 60: 1610–1615.
Koller W, Vetere-Overfield B, Gray C, Alexander C, et al. Environmental risk factors in Parkinson's disease. Neurology 1990; 40: 1218–1221.
Firestone JA, Smith-Weller T, Franklin G, Swanson P, et al. Pesticides and risk of Parkinson disease: a population-based case-control study. Arch Neurol 2005; 62: 91–95.
Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S . A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology 2000; 21: 435–440.
Ascherio A, Chen H, Weisskopf MG, O'Reilly E, et al. Pesticide exposure and risk for Parkinson's disease. Ann Neurol 2006; 60: 197–203.
Baldereschi M, Inzitari M, Vanni P, Di Carlo A, et al. Pesticide exposure might be a strong risk factor for Parkinson's disease. Ann Neurol. In press.
Golbe LI, Farrell TM, Davis PH . Follow-up study of early-life protective and risk factors in Parkinson's disease. Mov Disord 1990; 5: 66–70.
Lai BC, Marion SA, Teschke K, Tsui JK . Occupational and environmental risk factors for Parkinson's disease. Parkinsonism Relat Disord 2002; 8: 297–309.
Langston JW, Ballard P, Tetrud JW, Irwin I . Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983; 219: 979–980.
Schapira AH, Cooper JM, Dexter D, Jenner P, et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1989; 1: 1269.
Betarbet R, Sherer TB, Greenamyre JT . Animal models of Parkinson's disease. Bioessays 2002; 24: 308–318.
Calne DB, Langston JW, Martin WR, Stoessl AJ, et al. Positron emission tomography after MPTP: observations relating to the cause of Parkinson's disease. Nature 1985; 317: 246–248.
Kuopio A, Marttila RJ, Helenius H, Rinne UK . Familial occurrence of Parkinson's disease in a community-based case-control study. Parkinsonism Relat Disord 2001; 7: 297–303.
Payami H, Larsen K, Bernard S, Nutt J . Increased risk of Parkinson's disease in parents and siblings of patients. Ann Neurol 1994; 36: 659–661.
Autere JM, Moilanen JS, Myllyla VV, Majamaa K . Familial aggregation of Parkinson's disease in a Finnish population. J Neurol Neurosurg Psychiatry 2000; 69: 107–109.
Sveinbjornsdottir S, Hicks AA, Jonsson T, Petursson H, et al. Familial aggregation of Parkinson's disease in Iceland. N Engl J Med 2000; 343: 1765–1770.
Preux PM, Condet A, Anglade C, Druet-Cabanac M, et al. Parkinson's disease and environmental factors. Matched case-control study in the Limousin region, France. Neuroepidemiology 2000; 19: 333–337.
Elbaz A, Grigoletto F, Baldereschi M, Breteler MM, et al. Familial aggregation of Parkinson's disease: a population-based case-control study in Europe. EUROPARKINSON Study Group. Neurology 1999; 52: 1876–1882.
Uitti RJ, Shinotoh H, Hayward M, Schulzer M, et al. “Familial Parkinson's disease”—a case-control study of families. Can J Neurol Sci 1997; 24: 127–132.
De Michele G, Filla A, Marconi R, Volpe G, et al. A genetic study of Parkinson's disease. J Neural Transm Suppl 1995; 45: 21–25.
Vieregge P . Genetic factors in the etiology of idiopathic Parkinson's disease. J Neural Transm Park Dis Dement Sect 1994; 8: 1–37.
Forno LS . Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol 1996; 55: 259–272.
Braak H, Braak E . Pathoanatomy of Parkinson's disease. J Neurol 2000; 247( suppl 2): II3–II10.
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ . The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002; 125: 861–870.
Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ . What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. 1992. Neurology 2001; 57: S34–38.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 1997; 276: 2045–2047.
Bostantjopoulou S, Katsarou Z, Papadimitriou A, Veletza V, et al. Clinical features of parkinsonian patients with the alpha-synuclein (G209A) mutation. Mov Disord 2001; 16: 1007–1013.
Gasser T . Genetics of Parkinson's disease. J Neurol 2001; 248: 833–840.
Kruger R, Kuhn W, Muller T, Woitalla D, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet 1998; 18: 106–108.
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 2004; 55: 164–173.
Chan P, Jiang X, Forno LS, Di Monte DA, et al. Absence of mutations in the coding region of the alpha-synuclein gene in pathologically proven Parkinson's disease. Neurology 1998; 50: 1136–1137.
Farrer M, Wavrant-De Vrieze F, Crook R, Boles L, et al. Low frequency of alpha-synuclein mutations in familial Parkinson's disease. Ann Neurol 1998; 43: 394–397.
Vaughan JR, Farrer MJ, Wszolek ZK, Gasser T, et al. Sequencing of the alpha-synuclein gene in a large series of cases of familial Parkinson's disease fails to reveal any further mutations. The European Consortium on Genetic Susceptibility in Parkinson's Disease (GSPD). Hum Mol Genet 1998; 7: 751–753.
El-Agnaf OM, Curran MD, Wallace A, Middleton D, et al. Mutation screening in exons 3 and 4 of alpha-synuclein in sporadic Parkinson's and sporadic and familial dementia with Lewy bodies cases. Neuroreport 1998; 9: 3925–3927.
Pastor P, Munoz E, Ezquerra M, Obach V, et al. Analysis of the coding and the 5′ flanking regions of the alpha-synuclein gene in patients with Parkinson's disease. Mov Disord 2001; 16: 1115–1119.
Hope AD, Myhre R, Kachergus J, Lincoln S, et al. Alpha-synuclein missense and multiplication mutations in autosomal dominant Parkinson's disease. Neurosci Lett 2004; 367: 97–100.
Berg D, Niwar M, Maass S, Zimprich A, et al. Alpha-synuclein and Parkinson's disease: implications from the screening of more than 1,900 patients. Mov Disord 2005; 20: 1191–1194.
Singleton AB, Farrer M, Johnson J, Singleton A, et al. Alpha-synuclein locus triplication causes Parkinson's disease. Science 2003; 302: 841.
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 2004; 364: 1167–1169.
Ibanez P, Bonnet AM, Debarges B, Lohmann E, et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet 2004; 364: 1169–1171.
Nishioka K, Hayashi S, Farrer MJ, Singleton AB, et al. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson's disease. Ann Neurol 2006; 59: 298–309.
Johnson J, Hague SM, Hanson M, Gibson A, et al. SNCA multiplication is not a common cause of Parkinson disease or dementia with Lewy bodies. Neurology 2004; 63: 554–556.
Hofer A, Berg D, Asmus F, Niwar M, et al. The role of alpha-synuclein gene multiplications in early-onset Parkinson's disease and dementia with Lewy bodies. J Neural Transm 2005; 112: 1249–1254.
Gispert S, Trenkwalder C, Mota-Vieira L, Kostic V, et al. Failure to find alpha-synuclein gene dosage changes in 190 patients with familial Parkinson disease. Arch Neurol 2005; 62: 96–98.
Ahn TB, Kim SY, Kim JY, Park SS, et al. {alpha}-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. In press.
Kruger R, Vieira-Saecker AM, Kuhn W, Berg D, et al. Increased susceptibility to sporadic Parkinson's disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann Neurol 1999; 45: 611–617.
Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA 2006; 296: 661–670.
Chiba-Falek O, Kowalak JA, Smulson ME, Nussbaum RL . Regulation of alpha-synuclein expression by poly (ADP ribose) polymerase-1 (PARP-1) binding to the NACP-Rep1 polymorphic site upstream of the SNCA gene. Am J Hum Genet 2005; 76: 478–492.
Zimprich A, Biskup S, Leitner P, Lichtner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004; 44: 601–607.
Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 2004; 44: 595–600.
Di Fonzo A, Rohe CF, Ferreira J, Chien HF, et al. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson's disease. Lancet 2005; 365: 412–415.
Nichols WC, Pankratz N, Hernandez D, Paisan-Ruiz C, et al. Genetic screening for a single common LRRK2 mutation in familial Parkinson's disease. Lancet 2005; 365: 410–412.
Kachergus J, Mata IF, Hulihan M, Taylor JP, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet 2005; 76: 672–680.
Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet 2005; 365: 415–416.
Tan EK, Shen H, Tan LC, Farrer M, et al. The G2019S LRRK2 mutation is uncommon in an Asian cohort of Parkinson's disease patients. Neurosci Lett 2005; 384: 327–329.
Cho JW, Kim SY, Park SS, Kim HJ, et al. The G2019S LRRK2 mutation is rare in Korean patients with Parkinson's disease. Can J Neurol Sci 2007; 34: 53–55.
Lesage S, Ibanez P, Lohmann E, Pollak P, et al. G2019S LRRK2 mutation in French and North African families with Parkinson's disease. Ann Neurol 2005; 58: 784–787.
Goldwurm S, Zini M, Mariani L, Tesei S, et al. Evaluation of LRRK2 G2019S penetrance: relevance for genetic counseling in Parkinson disease. Neurology 2007; 68: 1141–1143.
Ozelius LJ, Senthil G, Saunders-Pullman R, Ohmann E, et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med 2006; 354: 424–425.
Lesage S, Durr A, Tazir M, Lohmann E, et al. LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. N Engl J Med 2006; 354: 422–423.
Ishihara L, Gibson RA, Warren L, Amouri R, et al. Screening for LRRK2 G2019S and clinical comparison of Tunisian and North American Caucasian Parkinson's disease families. Mov Disord 2007; 22: 55–61.
Bras JM, Guerreiro RJ, Ribeiro MH, Januario C, et al. G2019S dardarin substitution is a common cause of Parkinson's disease in a Portuguese cohort. Mov Disord 2005; 20: 1653–1655.
Ferreira JJ, Guedes LC, Rosa MM, Coelho M, et al. High prevalence of LRRK2 mutations in familial and sporadic Parkinson's disease in Portugal. Mov Disord 2007; 22: 1194–1201.
Ishihara L, Warren L, Gibson R, Amouri R, et al. Clinical features of Parkinson disease patients with homozygous leucine-rich repeat kinase 2 G2019S mutations. Arch Neurol 2006; 63: 1250–1254.
Adams JR, van Netten H, Schulzer M, Mak E, et al. PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation. Brain 2005; 128: 2777–2785.
Ross OA, Toft M, Whittle AJ, Johnson JL, et al. LRRK2 and Lewy body disease. Ann Neurol 2006; 59: 388–393.
Rajput A, Dickson DW, Robinson CA, Ross OA, et al. Parkinsonism, LRRK2 G2019S, and tau neuropathology. Neurology 2006; 67: 1506–1508.
Dachsel JC, Ross OA, Mata IF, Kachergus J, et al. LRRK2 G2019S substitution in frontotemporal lobar degeneration with ubiquitin-immunoreactive neuronal inclusions. Acta Neuropathol (Berl) 2007; 113: 601–606.
Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, et al. LRRK2 in Parkinson's disease: protein domains and functional insights. Trends Neurosci 2006; 29: 286–293.
Berg D, Schweitzer K, Leitner P, Zimprich A, et al. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson's disease. Brain 2005; 128: 3000–3011.
Mata IF, Kachergus JM, Taylor JP, Lincoln S, et al. LRRK2 pathogenic substitutions in Parkinson's disease. Neurogenetics 2005; 6: 171–177.
Paisan-Ruiz C, Lang AE, Kawarai T, Sato C, et al. LRRK2 gene in Parkinson disease: mutation analysis and case control association study. Neurology 2005; 65: 696–700.
Di Fonzo A, Tassorelli C, De Mari M, Chien HF, et al. Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson's disease. Eur J Hum Genet 2006; 14: 322–331.
Nichols WC, Elsaesser VE, Pankratz N, Pauciulo MW, et al. LRRK2 mutation analysis in Parkinson disease families with evidence of linkage to PARK8. Neurology. In press.
Skipper L, Li Y, Bonnard C, Pavanni R, et al. Comprehensive evaluation of common genetic variation within LRRK2 reveals evidence for association with sporadic Parkinson's disease. Hum Mol Genet 2005; 14: 3549–3556.
Pankratz N, Pauciulo MW, Elsaesser VE, Marek DK, et al. Mutations in LRRK2 other than G2019S are rare in a north American-based sample of familial Parkinson's disease. Mov Disord 2006; 21: 2257–2260.
Nichols WC, Marek DK, Pauciulo MW, Pankratz N, et al. R1514Q substitution in LRRK2 is not a pathogenic Parkinson's disease mutation. Mov Disord 2007; 22: 254–257.
West AB, Moore DJ, Biskup S, Bugayenko A, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA 2005; 102: 16842–16847.
Smith WW, Pei Z, Jiang H, Moore DJ, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci USA 2005; 102: 18676–18681.
Smith WW, Pei Z, Jiang H, Dawson VL, et al. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 2006; 9: 1231–1233.
Gasser T, Muller-Myhsok B, Wszolek ZK, Oehlmann R, et al. A susceptibility locus for Parkinson's disease maps to chromosome 2p13. Nat Genet 1998; 18: 262–265.
Karamohamed S, DeStefano AL, Wilk JB, Shoemaker CM, et al. A haplotype at the PARK3 locus influences onset age for Parkinson's disease: the GenePD study. Neurology 2003; 61: 1557–1561.
Sharma M, Mueller JC, Zimprich A, Lichtner P, et al. The sepiapterin reductase gene region reveals association in the PARK3 locus: analysis of familial and sporadic Parkinson's disease in European populations. J Med Genet 2006; 43: 557–562.
West AB, Zimprich A, Lockhart PJ, Farrer M, et al. Refinement of the PARK3 locus on chromosome 2p13 and the analysis of 14 candidate genes. Eur J Hum Genet 2001; 9: 659–666.
Steinberger D, Blau N, Goriuonov D, Bitsch J, et al. Heterozygous mutation in 5′-untranslated region of sepiapterin reductase gene (SPR) in a patient with dopa-responsive dystonia. Neurogenetics 2004; 5: 187–190.
Leroy E, Boyer R, Auburger G, Leube B, et al. The ubiquitin pathway in Parkinson's disease. Nature 1998; 395: 451–452.
Healy DG, Abou-Sleiman PM, Wood NW . Genetic causes of Parkinson's disease: UCHL-1. Cell Tissue Res 2004; 318: 189–194.
Healy DG, Abou-Sleiman PM, Casas JP, Ahmadi KR, et al. UCHL-1 is not a Parkinson's disease susceptibility gene. Ann Neurol 2006; 59: 627–633.
Kitada T, Asakawa S, Hattori N, Matsumine H, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998; 392: 605–608.
Lohmann E, Periquet M, Bonifati V, Wood NW, et al. How much phenotypic variation can be attributed to parkin genotype?. Ann Neurol 2003; 54: 176–185.
Bonifati V, De Michele G, Lucking CB, Durr A, et al. The parkin gene and its phenotype. Italian PD Genetics Study Group, French PD Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Neurol Sci 2001; 22: 51–52.
Farrer M, Chan P, Chen R, Tan L, et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann Neurol 2001; 50: 293–300.
Hedrich K, Eskelson C, Wilmot B, Marder K, et al. Distribution, type, and origin of parkin mutations: review and case studies. Mov Disord 2004; 19: 1146–1157.
Lucking CB, Abbas N, Durr A, Bonifati V, et al. Homozygous deletions in parkin gene in European and North African families with autosomal recessive juvenile parkinsonism. The European Consortium on Genetic Susceptibility in Parkinson's Disease and the French Parkinson's Disease Genetics Study Group. Lancet 1998; 352: 1355–1356.
Abbas N, Lucking CB, Ricard S, Durr A, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson's Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Hum Mol Genet 1999; 8: 567–574.
Lucking CB, Durr A, Bonifati V, Vaughan J, et al. Association between early-onset Parkinson's disease and mutations in the parkin gene. N Engl J Med 2000; 342: 1560–1567.
Hedrich K, Marder K, Harris J, Kann M, et al. Evaluation of 50 probands with early-onset Parkinson's disease for parkin mutations. Neurology 2002; 58: 1239–1246.
Kann M, Jacobs H, Mohrmann K, Schumacher K, et al. Role of parkin mutations in 111 community-based patients with early-onset parkinsonism. Ann Neurol 2002; 51: 621–625.
Nichols WC, Pankratz N, Uniacke SK, Pauciulo MW, et al. Linkage stratification and mutation analysis at the parkin locus identifies mutation positive Parkinson's disease families. J Med Genet 2002; 39: 489–492.
West A, Periquet M, Lincoln S, Lucking CB, et al. Complex relationship between parkin mutations and Parkinson disease. Am J Med Genet 2002; 114: 584–591.
Foroud T, Uniacke SK, Liu L, Pankratz N, et al. Heterozygosity for a mutation in the parkin gene leads to later onset Parkinson disease. Neurology 2003; 60: 796–801.
Periquet M, Lucking C, Vaughan J, Bonifati V, et al. Origin of the mutations in the parkin gene in Europe: exon rearrangements are independent recurrent events, whereas point mutations may result from founder effects. Am J Hum Genet 2001; 68: 617–626.
Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem 2003; 278: 43628–43635.
Klein C, Schumacher K, Jacobs H, Hagenah J, et al. Association studies of Parkin-son's disease and parkin polymorphisms. Ann Neurol 2000; 48: 126–127.
Sun M, Latourelle JC, Wooten GF, Lew MF, et al. Influence of heterozygosity for parkin mutation on onset age in familial Parkinson disease: the GenePD study. Arch Neurol 2006; 63: 826–832.
Kay DM, Moran D, Moses L, Poorkaj P, et al. Heterozygous parkin point mutations are as common in control subjects as in Parkinson's patients. Ann Neurol 2007; 61: 47–54.
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 2004; 304: 1158–1160.
Hatano Y, Li Y, Sato K, Asakawa S, et al. Novel PINK1 mutations in early-onset parkinsonism. Ann Neurol 2004; 56: 424–427.
Healy DG, Abou-Sleiman PM, Gibson JM, Ross OA, et al. PINK1 (PARK6) associated Parkinson disease in Ireland. Neurology 2004; 63: 1486–1488.
Li Y, Tomiyama H, Sato K, Hatano Y, et al. Clinicogenetic study of PINK1 mutations in autosomal recessive early-onset parkinsonism. Neurology 2005; 64: 1955–1957.
Bonifati V, Rohe CF, Breedveld GJ, Fabrizio E, et al. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology 2005; 65: 87–95.
Steinlechner S, Stahlberg J, Volkel B, Djarmati A, et al. Co-occurrence of affective and schizophrenia spectrum disorders with PINK1 mutations. J Neurol Neurosurg Psychiatry 2007; 78: 532–535.
Ephraty L, Porat O, Israeli D, Cohen OS, et al. Neuropsychiatric and cognitive features in autosomal-recessive early parkinsonism due to PINK1 mutations. Mov Disord 2007; 22: 566–569.
Rogaeva E, Johnson J, Lang AE, Gulick C, et al. Analysis of the PINK1 gene in a large cohort of cases with Parkinson disease. Arch Neurol 2004; 61: 1898–1904.
Clark IE, Dodson MW, Jiang C, Cao JH, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006; 441: 1162–1166.
Abou-Sleiman PM, Muqit MM, McDonald NQ, Yang YX, et al. A heterozygous effect for PINK1 mutations in Parkinson's disease?. Ann Neurol 2006; 60: 414–419.
Tang B, Xiong H, Sun P, Zhang Y, et al. Association of PINK1 and DJ-1 confers digenic inheritance of early-onset Parkinson's disease. Hum Mol Genet 2006; 15: 1816–1825.
Djarmati A, Hedrich K, Svetel M, Lohnau T, et al. Heterozygous PINK1 mutations: a susceptibility factor for Parkinson disease?. Mov Disord 2006; 21: 1526–1530.
Toft M, Myhre R, Pielsticker L, White LR, et al. PINK1 mutation heterozygosity and the risk of Parkinson's disease. J Neurol Neurosurg Psychiatry 2007; 78: 82–84.
Bonifati V, Rizzu P, van Baren MJ, Schaap O, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003; 299: 256–259.
Hague S, Rogaeva E, Hernandez D, Gulick C, et al. Early-onset Parkinson's disease caused by a compound heterozygous DJ-1 mutation. Ann Neurol 2003; 54: 271–274.
Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, et al. The role of pathogenic DJ-1 mutations in Parkinson's disease. Ann Neurol 2003; 54: 283–286.
Djarmati A, Hedrich K, Svetel M, Schafer N, et al. Detection of parkin (PARK2) and DJ1 (PARK7) mutations in early-onset Parkinson disease: parkin mutation frequency depends on ethnic origin of patients. Hum Mutat 2004; 23: 525
Hering R, Strauss KM, Tao X, Bauer A, et al. Novel homozygous p.E64D mutation in DJ1 in early onset Parkinson disease (PARK7). Hum Mutat 2004; 24: 321–329.
Annesi G, Savettieri G, Pugliese P, D'Amelio M, et al. DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral sclerosis complex. Ann Neurol 2005; 58: 803–807.
Ibanez P, De Michele G, Bonifati V, Lohmann E, et al. Screening for DJ-1 mutations in early onset autosomal recessive parkinsonism. Neurology 2003; 61: 1429–1431.
Lockhart PJ, Bounds R, Hulihan M, Kachergus J, et al. Lack of mutations in DJ-1 in a cohort of Taiwanese ethnic Chinese with early-onset parkinsonism. Mov Disord 2004; 19: 1065–1069.
Lockhart PJ, Lincoln S, Hulihan M, Kachergus J, et al. DJ-1 mutations are a rare cause of recessively inherited early onset parkinsonism mediated by loss of protein function. J Med Genet 2004; 41: e22.
Clark LN, Afridi S, Mejia-Santana H, Harris J, et al. Analysis of an early-onset Parkinson's disease cohort for DJ-1 mutations. Mov Disord 2004; 19: 796–800.
Mitsumoto A, Nakagawa Y . DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res 2001; 35: 885–893.
Taira T, Saito Y, Niki T, Iguchi-Ariga SM, et al. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep 2004; 5: 213–218.
Kinumi T, Kimata J, Taira T, Ariga H, et al. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem Biophys Res Commun 2004; 317: 722–728.
Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA 2004; 101: 9103–9108.
Shendelman S, Jonason A, Martinat C, Leete T, et al. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol 2004; 2: e362.
Zhou W, Zhu M, Wilson MA, Petsko GA, et al. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J Mol Biol 2006; 356: 1036–1048.
Waragai M, Wei J, Fujita M, Nakai M, et al. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson's disease. Biochem Biophys Res Commun 2006; 345: 967–972.
Chen L, Cagniard B, Mathews T, Jones S, et al. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J Biol Chem 2005; 280: 21418–21426.
DeStefano AL, Golbe LI, Mark MH, Lazzarini AM, et al. Genome-wide scan for Parkinson's disease: the GenePD Study. Neurology 2001; 57: 1124–1126.
Pankratz N, Nichols WC, Uniacke SK, Halter C, et al. Genome screen to identify susceptibility genes for Parkinson disease in a sample without parkin mutations. Am J Hum Genet 2002; 71: 124–135.
Li YJ, Scott WK, Hedges DJ, Zhang F, et al. Age at onset in two common neurodegenerative diseases is genetically controlled. Am J Hum Genet 2002; 70: 985–993.
DeStefano AL, Lew MF, Golbe LI, Mark MH, et al. PARK3 influences age at onset in Parkinson disease: a genome scan in the GenePD study. Am J Hum Genet 2002; 70: 1089–1095.
Pankratz N, Nichols WC, Uniacke SK, Halter C, et al. Genome-wide linkage analysis and evidence of gene-by-gene interactions in a sample of 362 multiplex Parkinson disease families. Hum Mol Genet 2003; 12: 2599–2608.
Pankratz N, Uniacke SK, Halter CA, Rudolph A, et al. Genes influencing Parkinson disease onset: replication of PARK3 and identification of novel loci. Neurology 2004; 62: 1616–1618.
Martinez M, Brice A, Vaughan JR, Zimprich A, et al. Genome-wide scan linkage analysis for Parkinson's disease: the European genetic study of Parkinson's disease. J Med Genet 2004; 41: 900–907.
Foroud T, Pankratz N, Martinez M . Chromosome 5 and Parkinson disease. Eur J Hum Genet 2006; 14: 1106–1110.
Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, et al. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet 2005; 77: 685–693.
Fung HC, Scholz S, Matarin M, Simon-Sanchez J, et al. Genome-wide genotyping in Parkinson's disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol 2006; 5: 911–916.
Clarimon J, Scholz S, Fung HC, Hardy J, et al. Conflicting results regarding the semaphorin gene (SEMA5A) and the risk for Parkinson disease. Am J Hum Genet 2006; 78: 1082–1084.
Farrer MJ, Haugarvoll K, Ross OA, Stone JT, et al. Genomewide association, Parkinson disease, and PARK10. Am J Hum Genet 2006; 78: 1084–1088.
Goris A, Williams-Gray CH, Foltynie T, Compston DA, et al. No evidence for association with Parkinson disease for 13 single-nucleotide polymorphisms identified by whole-genome association screening. Am J Hum Genet 2006; 78: 1088–1090.
Myers RH . Considerations for genomewide association studies in Parkinson disease. Am J Hum Genet 2006; 78: 1081–1082.
Tan EK, Khajavi M, Thornby JI, Nagamitsu S, et al. Variability and validity of polymorphism association studies in Parkinson's disease. Neurology 2000; 55: 533–538.
Law SW, Conneely OM, DeMayo FJ, O'Malley BW . Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol 1992; 6: 2129–2135.
Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA 1998; 95: 4013–4018.
Castillo SO, Baffi JS, Palkovits M, Goldstein DS, et al. Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci 1998; 11: 36–46.
Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science 1997; 276: 248–250.
Le W, Conneely OM, Zou L, He Y, et al. Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp Neurol 1999; 159: 451–458.
Le W, Conneely OM, He Y, Jankovic J, et al. Reduced Nurr1 expression increases the vulnerability of mesencephalic dopamine neurons to MPTP-induced injury. J Neurochem 1999; 73: 2218–2221.
Le WD, Xu P, Jankovic J, Jiang H, et al. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet 2003; 33: 85–89.
Wellenbrock C, Hedrich K, Schafer N, Kasten M, et al. NR4A2 mutations are rare among European patients with familial Parkinson's disease. Ann Neurol 2003; 54: 415
Zimprich A, Asmus F, Leitner P, Castro M, et al. Point mutations in exon 1 of the NR4A2 gene are not a major cause of familial Parkinson's disease. Neurogenetics 2003; 4: 219–220.
Hering R, Petrovic S, Mietz EM, Holzmann C, et al. Extended mutation analysis and association studies of Nurr1 (NR4A2) in Parkinson disease. Neurology 2004; 62: 1231–1232.
Nichols WC, Uniacke SK, Pankratz N, Reed T, et al. Evaluation of the role of Nurr1 in a large sample of familial Parkinson's disease. Mov Disord 2004; 19: 649–655.
Ibanez P, Lohmann E, Pollak P, Durif F, et al. Absence of NR4A2 exon 1 mutations in 108 families with autosomal dominant Parkinson disease. Neurology 2004; 62: 2133–2134.
Karamohamed S, Golbe LI, Mark MH, Lazzarini AM, et al. Absence of previously reported variants in the SCNA (G88C and G209A), NR4A2 (T291D and T245G) and the DJ-1 (T497C) genes in familial Parkinson's disease from the GenePD study. Mov Disord 2005; 20: 1188–1191.
Healy DG, Abou-Sleiman PM, Ahmadi KR, Gandhi S, et al. NR4A2 genetic variation in sporadic Parkinson's disease: a genewide approach. Mov Disord 2006; 21: 1960–1963.
Marx FP, Holzmann C, Strauss KM, Li L, et al. Identification and functional characterization of a novel R621C mutation in the synphilin-1 gene in Parkinson's disease. Hum Mol Genet 2003; 12: 1223–1231.
Farrer M, Destee A, Levecque C, Singleton A, et al. Genetic analysis of synphilin-1 in familial Parkinson's disease. Neurobiol Dis 2001; 8: 317–323.
Bandopadhyay R, de Silva R, Khan N, Graham E, et al. No pathogenic mutations in the synphilin-1 gene in Parkinson's disease. Neurosci Lett 2001; 307: 125–127.
Maraganore DM, Farrer MJ, Lesnick TG, de Andrade M, et al. Case-control study of the alpha-synuclein interacting protein gene and Parkinson's disease. Mov Disord 2003; 18: 1233–1239.
Slooter AJ, Cruts M, Kalmijn S, Hofman A, et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol 1998; 55: 964–968.
Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, Dubois B, et al. Motor signs during the course of Alzheimer disease. Neurology 2004; 63: 975–982.
Harhangi BS, de Rijk MC, van Duijn CM, Van Broeckhoven C, et al. APOE and the risk of PD with or without dementia in a population-based study. Neurology 2000; 54: 1272–1276.
Li YJ, Hauser MA, Scott WK, Martin ER, et al. Apolipoprotein E controls the risk and age at onset of Parkinson disease. Neurology 2004; 62: 2005–2009.
Ghebremedhin E, Del Tredici K, Vuksic M, Rub U, et al. Relationship of apolipoprotein E and age at onset to Parkinson disease neuropathology. J Neuropathol Exp Neurol 2006; 65: 116–123.
Papapetropoulos S, Farrer MJ, Stone JT, Milkovic NM, et al. Phenotypic associations of tau and ApoE in Parkinson's disease. Neurosci Lett 2007; 414: 141–144.
Lopez M, Guerrero J, Yescas P, Boll MC, et al. Apolipoprotein E epsilon4 allele is associated with Parkinson disease risk in a Mexican Mestizo population. Mov Disord 2007; 22: 417–420.
Huang X, Chen PC, Poole C . APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology 2004; 62: 2198–2202.
Huang X, Chen P, Kaufer DI, Troster AI, et al. Apolipoprotein E and dementia in Parkinson disease: a meta-analysis. Arch Neurol 2006; 63: 189–193.
Pankratz N, Byder L, Halter C, Rudolph A, et al. Presence of an APOE4 allele results in significantly earlier onset of Parkinson's disease and a higher risk with dementia. Mov Disord 2006; 21: 45–49.
Zareparsi S, Camicioli R, Sexton G, Bird T, et al. Age at onset of Parkinson disease and apolipoprotein E genotypes. Am J Med Genet 2002; 107: 156–161.
Buchanan DD, Silburn PA, Prince JA, Mellick GD . Association of APOE with Parkinson disease age-at-onset in women. Neurosci Lett 2007; 411: 185–188.
Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R . Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med 2004; 351: 1972–1977.
Ziegler SG, Eblan MJ, Gutti U, Hruska KS, et al. Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Mol Genet Metab 2007; 91: 195–200.
Sato C, Morgan A, Lang AE, Salehi-Rad S, et al. Analysis of the glucocerebrosidase gene in Parkinson's disease. Mov Disord 2005; 20: 367–370.
Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, et al. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab 2004; 81: 70–73.
Goker-Alpan O, Giasson BI, Eblan MJ, Nguyen J, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology 2006; 67: 908–910.
Eblan MJ, Nguyen J, Ziegler SG, Lwin A, et al. Glucocerebrosidase mutations are also found in subjects with early-onset parkinsonism from Venezuela. Mov Disord 2006; 21: 282–283.
Toft M, Pielsticker L, Ross OA, Aasly JO, et al. Glucocerebrosidase gene mutations and Parkinson disease in the Norwegian population. Neurology 2006; 66: 415–417.
Rademakers R, Cruts M, van Broeckhoven C . The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 2004; 24: 277–295.
Giasson BI, Forman MS, Higuchi M, Golbe LI, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 2003; 300: 636–640.
Healy DG, Abou-Sleiman PM, Lees AJ, Casas JP, et al. Tau gene and Parkinson's disease: a case-control study and meta-analysis. J Neurol Neurosurg Psychiatry 2004; 75: 962–965.
Zhang J, Song Y, Chen H, Fan D . The tau gene haplotype h1 confers a susceptibility to Parkinson's disease. Eur Neurol 2005; 53: 15–21.
Zabetian CP, Hutter CM, Factor SA, Nutt JG, et al. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson's disease. Ann Neurol. In press.
Swerdlow RH, Parks JK, Miller SW, Tuttle JB, et al. Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol 1996; 40: 663–671.
Gu M, Cooper JM, Taanman JW, Schapira AH . Mitochondrial DNA transmission of the mitochondrial defect in Parkinson's disease. Ann Neurol 1998; 44: 177–186.
Ikebe S, Tanaka M, Ozawa T . Point mutations of mitochondrial genome in Parkinson's disease. Brain Res Mol Brain Res 1995; 28: 281–295.
Vives-Bauza C, Andreu AL, Manfredi G, Beal MF, et al. Sequence analysis of the entire mitochondrial genome in Parkinson's disease. Biochem Biophys Res Commun 2002; 290: 1593–1601.
van der Walt JM, Nicodemus KK, Martin ER, Scott WK, et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet 2003; 72: 804–811.
Ghezzi D, Marelli C, Achilli A, Goldwurm S, et al. Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson's disease in Italians. Eur J Hum Genet 2005; 13: 748–752.
Pyle A, Foltynie T, Tiangyou W, Lambert C, et al. Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol 2005; 57: 564–567.
Bender A, Krishnan KJ, Morris CM, Taylor GA, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 2006; 38: 515–517.
Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 2006; 38: 518–520.
Acknowledgements
This work was supported by NIH Grant R01 NS37167.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pankratz, N., Foroud, T. Genetics of Parkinson disease. Genet Med 9, 801–811 (2007). https://doi.org/10.1097/GIM.0b013e31815bf97c
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e31815bf97c
Keywords
This article is cited by
-
Neuronal activity modulates alpha-synuclein aggregation and spreading in organotypic brain slice cultures and in vivo
Acta Neuropathologica (2020)
-
Silencing of PINK1 Inhibits Insulin-Like Growth Factor-1-Mediated Receptor Activation and Neuronal Survival
Journal of Molecular Neuroscience (2015)
-
The effect of cannabis on oxidative stress and neurodegeneration induced by intrastriatal rotenone injection in rats
Comparative Clinical Pathology (2015)
-
Effect of a single intrastriatal rotenone injection on oxidative stress and neurodegeneration in the rat brain
Comparative Clinical Pathology (2014)
-
New Insight into Neurodegeneration: the Role of Proteomics
Molecular Neurobiology (2014)