Abstract
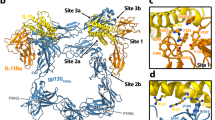
Interleukin-21 (IL-21) is a class I cytokine that belongs to the γc-subfamily of cytokines and regulates immune responses. It signals through a heterodimeric receptor complex composed of the IL-21R1 and γc-receptor chains. A characteristic feature of class I cytokine receptors is the presence of a consensus motif WSXWS (WS motif) in the membrane proximal fibronectin type III domain (FNIII) of these receptors. We recently described the structure of the IL-21R:IL-21 complex and showed that the first tryptophan of the WS motif of IL-21R is mannosylated and involved in formation of a sugar bridge that connects the two FNIII domains of the receptor. Furthermore, a mutation within the WS motif of IL-21R was recently shown to cause a novel kind of primary immunodeficiency syndrome (PID). Here, we report the structure of IL-21R alone, which shows that the sugar bridge forms independently of whether IL-21R binds IL-21 or not, and we furthermore investigate the role of this bridge in the export of IL-21R and γC to the plasma membrane. Thus, we provide a molecular explanation for how mutations in the WS motif may cause PIDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408: 57–63.
Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol 2004; 173: 68–78.
Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon-producing Th1 cells. J Exp Med 2002; 196: 969–977.
Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 2007; 178: 2827–2834.
Wei L, Laurence A, Elias KM, O’Shea JJ . IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 2007; 282: 34605–34610.
Parrish-Novak J, Foster DC, Holly RD, Clegg CH . Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol 2002; 72: 856–863.
Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med 2005; 201: 139–148.
Søndergaard H, Skak K . IL-21: roles in immunopathology and cancer therapy. Tissue Antigens 2009; 74: 467–479.
Hashmi MH, Van Veldhuizen PJ . Interleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent non-Hodgkin’s lymphoma. Expert Opin Biol Ther 2010; 10: 807–817.
Ozaki K, Spolski R, Feng CG, Qi C-F, Cheng J, Sher A et al. A critical role for IL-21 in regulating immunoglobulin production. Science 2002; 298: 1630–1634.
Iannello A, Boulassel M-R, Samarani S, Tremblay C, Toma E, Routy J-P et al. IL-21 enhances NK cell functions and survival in healthy and HIV-infected patients with minimal stimulation of viral replication. J Leukoc Biol 2010; 87: 857–867.
Chevalier MF, Jülg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol 2011; 85: 733–741.
Fröhlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 2009; 324: 1576–1580.
Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol 2011; 85: 2316–2324.
Ozaki K, Leonard WJ . Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem 2002; 277: 29355–29358.
Boulay J-L, O’Shea JJ, Paul WE . Molecular phylogeny within type I cytokines and their cognate receptors. Immunity 2003; 19: 159–163.
Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J 1995; 14: 3654–3663.
Hamming OJ, Kang L, Svensson A, Karlsen JL, Rahbek-Nielsen H, Paludan SR et al. Crystal structure of interleukin-21 receptor (IL-21R) bound to IL-21 reveals that sugar chain interacting with WSXWS motif is integral part of IL-21R. J Biol Chem 2012; 287: 9454–9460.
Weidemann T, Höfinger S, Müller K, Auer M . Beyond dimerization: a membrane-dependent activation model for interleukin-4 receptor-mediated signalling. J Mol Biol 2007; 366: 1365–1373.
Dagil R, Knudsen MJ, Olsen JG, O’Shea C, Franzmann M, Goffin V et al. The WSXWS motif in cytokine receptors is a molecular switch involved in receptor activation: insight from structures of the prolactin receptor. Structure 2012; 20: 270–282.
Hilton DJ, Watowich SS, Katz L, Lodish HF . Saturation mutagenesis of the WSXWS motif of the erythropoietin receptor. J Biol Chem 1996; 271: 4699–4708.
Miyazaki T, Maruyama M, Yamada G, Hatakeyama M, Taniguchi T . The integrity of the conserved ‘WS motif’ common to IL-2 and other cytokine receptors is essential for ligand binding and signal transduction. EMBO J 1991; 10: 3191–3197.
Chiba T, Amanuma H, Todokoro K . Tryptophan residue of TRP-SER-X-TRP-SER motif in domains of erythropoietin receptor is essential for signal transduction. Biochem Biophys Res Commun 1992; 184: 485–490.
Quelle DE, Quelle FW, Wojchowski DM . Mutations in the WSAWSE and cytosolic domains of the erythropoietin receptor affect signal transduction and ligand binding and internalization. Mol Cell Biol 1992; 12: 4553–4561.
Baumgartner JW, Wells Ca, Chen CM, Waters MJ . The role of the WSXWS equivalent motif in growth hormone receptor function. J Biol Chem 1994; 269: 29094–29101.
Wang X, Rickert M, Garcia KC . Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science 2005; 310: 1159–1163.
LaPorte SL, Juo ZS, Vaclavikova J, Colf La, Qi X, Heller NM et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 2008; 132: 259–272.
Bravo J, Staunton D, Heath JK, Jones EY . Crystal structure of a cytokine-binding region of gp130. EMBO J 1998; 17: 1665–1674.
Hage T, Sebald W, Reinemer P . Crystal structure of the interleukin-4/receptor alpha chain complex reveals a mosaic binding interface. Cell 1999; 97: 271–281.
Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 2008; 134: 496–507.
Yao C-M, Han X-H, Zhang Y-D, Zhang H, Jin Y-Y, Cao R-M et al. Clinical characteristics and genetic profiles of 44 patients with severe combined immunodeficiency (SCID): report from Shanghai, China (2004–2011). J Clin Immunol 2013; 33: 526–539.
Zhang C, Zhang Z-Y, Wu J-F, Tang X-M, Yang X-Q, Jiang L-P et al. Clinical characteristics and mutation analysis of X-linked severe combined immunodeficiency in China. World J Pediatr 2013; 9: 42–47.
Puck JM, Pepper AE, Henthorn PS, Candotti F, Isakov J, Whitwam T et al. Mutation analysis of IL2RG in human X-linked severe combined immunodeficiency. Blood 1997; 89: 1968–1977.
Kotlarz D, Ziętara N, Uzel G, Weidemann T, Braun CJ, Diestelhorst J et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med 2013; 210: 433–443.
Fokkema IF A C, Taschner PEM, Schaafsma GCP, Celli J, Laros JFJ, den Dunnen JT . LOVD v.2.0: the next generation in gene variant databases. Hum Mutat 2011; 32: 557–563.
Ring AM, Lin J-X, Feng D, Mitra S, Rickert M, Bowman GR et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat Immunol 2012; 13: 1187–1195.
Chirifu M, Hayashi C, Nakamura T, Toma S, Shuto T, Kai H et al. Crystal structure of the IL-15-IL-15Ralpha complex, a cytokine-receptor unit presented in trans. Nat Immunol 2007; 8: 1001–1007.
McElroy CA, Dohm JA, Walsh ST . Structural and biophysical studies of the human IL-7/IL-7Ralpha complex. Structure 2009; 17: 54–65.
Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA . Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 1999; 283: 987–990.
Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 2010; 66: 213–221.
Emsley P, Cowtan K . Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004; 60: 2126–2132.
Vancha AR, Govindaraju S, Parsa KVL, Jasti M, González-García M, Ballestero RP . Use of polyethyleneimine polymer in cell culture as attachment factor and lipofection enhancer. BMC Biotechnol 2004; 4: 23.
Acknowledgements
We thank Siv A Hjorth and Kent Bondensgaard for help with purifying the IL-21R protein, as well as Lene Niemann Nejsum for advice on imaging. This work was supported by grants from the Danish Cancer Society and the Danish Council for Independent Research | Natural Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Genes and Immunity website
Rights and permissions
About this article
Cite this article
Siupka, P., Hamming, O., Kang, L. et al. A conserved sugar bridge connected to the WSXWS motif has an important role for transport of IL-21R to the plasma membrane. Genes Immun 16, 405–413 (2015). https://doi.org/10.1038/gene.2015.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2015.22
This article is cited by
-
Genomic Spectrum and Phenotypic Heterogeneity of Human IL-21 Receptor Deficiency
Journal of Clinical Immunology (2021)