Abstract
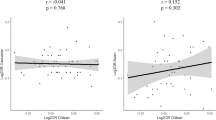
Expression of the major autoimmune risk loci DRB1 and DQB1 is regulated by the class II MHC (major histocompatibility complex) transactivator (CIITA), making the CIITA gene a strong autoimmune risk locus candidate. A CIITA promoter single-nucleotide polymorphism (SNP), rs3087456 (-168 A/G), has indeed been associated with several autoimmune diseases, including rheumatoid arthritis (RA). Recently, an intronic SNP rs8048002 has been suggested as a better susceptibility marker in Addison’s disease. Therefore, we tested both SNPs in a panel of autoimmune diseases, consisting of Norwegian patients with RA (n=819), juvenile idiopathic arthritis (JIA; n=524), or type 1 diabetes (T1D; n=1211), and 2149 controls. We also included an independent Swedish RA cohort (n=2503) and controls (n=1416). Both rs3087456 and rs8048002 were significantly associated with RA (combined Norwegian and Swedish patients Pcorrected=0.012 and Pcorrected=0.0016, respectively), but not with JIA or T1D. Meta-analysis of 16 RA cohorts confirmed rs3087456 with only marginal significance (P=0.016). However, results were stronger in the Scandinavian subgroup (4 cohorts, P=3.8 × 10−4), indicating a population-dependent effect. A similar pattern was observed in a meta-analysis of rs8048002. Our results support involvement of CIITA in RA, but imply that this is population dependent and that the aetiological variant is yet to be discovered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reith W, LeibundGut-Landmann S, Waldburger JM . Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol 2005; 5: 793–806.
Iikuni N, Ikari K, Momohara S, Tomatsu T, Hara M, Yamanaka H et al. MHC2TA is associated with rheumatoid arthritis in Japanese patients. Ann Rheum Dis 2007; 66: 274–275.
Orozco G, Robledo G, Linga Reddy MV, Garcia A, Pascual-Salcedo D, Balsa A et al. Study of the role of a functional polymorphism of MHC2TA in rheumatoid arthritis in three ethnically different populations. Rheumatology (Oxford) 2006; 45: 1442–1444.
Swanberg M, Lidman O, Padyukov L, Eriksson P, Akesson E, Jagodic M et al. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat Genet 2005; 37: 486–494.
Skinningsrud B, Husebye ES, Pearce SH, McDonald DO, Brandal K, Wolff AB et al. Polymorphisms in CLEC16A and CIITA at 16p13 are associated with primary adrenal insufficiency. J Clin Endocrinol Metab 2008; 93: 3310–3317.
Dieguez-Gonzalez R, Akar S, Calaza M, Gonzalez-Alvaro I, Fernandez-Gutierrez B, Lamas JR et al. Lack of association with rheumatoid arthritis of selected polymorphisms in 4 candidate genes: CFH, CD209, eotaxin-3, and MHC2TA. J Rheumatol 2009; 36: 1590–1595.
Eyre S, Bowes J, Spreckley K, Potter C, Ring S, Strachan D et al. Investigation of the MHC2TA gene, associated with rheumatoid arthritis in a Swedish population, in a UK rheumatoid arthritis cohort. Arthritis Rheum 2006; 54: 3417–3412.
Harrison P, Pointon JJ, Farrar C, Harin A, Wordsworth BP . MHC2TA promoter polymorphism (−168G/A, rs3087456) is not associated with susceptibility to rheumatoid arthritis in British Caucasian rheumatoid arthritis patients. Rheumatology (Oxford) 2007; 46: 409–411.
Newman WG, Zhang Q, Liu X, Walker E, Ternan H, Owen J et al. Rheumatoid arthritis association with the FCRL3 -169C polymorphism is restricted to PTPN22 1858T-homozygous individuals in a Canadian population. Arthritis Rheum 2006; 54: 3820–3827.
Plant D, Barton A, Thomson W, Ke X, Eyre S, Hinks A et al. A re-evaluation of three putative functional single nucleotide polymorphisms in rheumatoid arthritis. Ann Rheum Dis 2009; 68: 1373–1375.
Yazdani-Biuki B, Brickmann K, Wohlfahrt K, Mueller T, Marz W, Renner W et al. The MHC2TA -168A>G gene polymorphism is not associated with rheumatoid arthritis in Austrian patients. Arthritis Res Ther 2006; 8: R97.
Akkad DA, Jagiello P, Szyld P, Goedde R, Wieczorek S, Gross WL et al. Promoter polymorphism rs3087456 in the MHC class II transactivator gene is not associated with susceptibility for selected autoimmune diseases in German patient groups. Int J Immunogenet 2006; 33: 59–61.
O'Doherty C, Hawkins S, Rooney M, Vandenbroeck K . The MHC2TA-168A/G and +1614G/C polymorphisms and risk for multiple sclerosis or chronic inflammatory arthropathies. Tissue Antigens 2007; 70: 247–251.
Martinez A, Sanchez-Lopez M, Varade J, Mas A, Martin MC, de Las Heras V et al. Role of the MHC2TA gene in autoimmune diseases. Ann Rheum Dis 2007; 66: 325–329.
Bronson PG, Criswell LA, Barcellos LF . The MHC2TA −168A/G polymorphism and risk for rheumatoid arthritis: a meta-analysis of 6861 patients and 9270 controls reveals no evidence for association. Ann Rheum Dis 2008; 67: 933–936.
Martinez A, Perdigones N, Cenit MC, Espino L, Varade J, Lamas JR et al. Chromosomal region 16p13: further evidence of increased predisposition to immune diseases. Ann Rheum Dis 2010; 69: 309–311.
Bronson PG, Ramsay PP, Seldin MF, Gregersen PK, Criswell LA, Barcellos LF . CIITA is not associated with risk of developing rheumatoid arthritis. Genes Immun 2011; 12: 235–238.
Skinningsrud B, Lie BA, Husebye ES, Kvien TK, Forre O, Flato B et al. A CLEC16A variant confers risk for juvenile idiopathic arthritis and anti-CCP negative rheumatoid arthritis. Ann Rheum Dis 2010; 69: 1471–1474.
McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 2010; 42: 332–337.
Libert F, Cochaux P, Beckman G, Samson M, Aksenova M, Cao A et al. The deltaccr5 mutation conferring protection against HIV-1 in Caucasian populations has a single and recent origin in Northeastern Europe. Hum Mol Genet 1998; 7: 399–406.
van der Woude D, Lie BA, Lundstrom E, Balsa A, Feitsma AL, Houwing-Duistermaat JJ et al. Protection against anti-citrullinated protein antibody-positive rheumatoid arthritis is predominantly associated with HLA-DRB11301: a meta-analysis of HLA-DRB1 associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in four European populations. Arthritis Rheum 2010; 62: 1236–1245.
Vang T, Miletic AV, Bottini N, Mustelin T . Protein tyrosine phosphatase PTPN22 in human autoimmunity. Autoimmunity 2007; 40: 453–461.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324.
Kvien TK, Uhlig T . The Oslo experience with arthritis registries. Clin Exp Rheumatol 2003; 21(Suppl 31): S118–S122.
Syversen SW, Gaarder PI, Goll GL, Odegard S, Haavardsholm EA, Mowinckel P et al. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis 2008; 67: 212–217.
Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis 2003; 62: 835–841.
Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006; 54: 38–46.
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004; 31: 390–392.
EURODIAB ACE Study Group Variation and trends in incidence of childhood diabetes in Europe. Lancet 2000; 355: 873–876.
Barker DL, Hansen MS, Faruqi AF, Giannola D, Irsula OR, Lasken RS et al. Two methods of whole-genome amplification enable accurate genotyping across a 2320-SNP linkage panel. Genome Res 2004; 14(5): 901–907.
Sayer DC, Whidborne R, De Santis D, Rozemuller EH, Christiansen FT, Tilanus MG . A multicenter international evaluation of single-tube amplification protocols for sequencing-based typing of HLA-DRB1 and HLA-DRB3,4,5. Tissue Antigens 2004; 63: 412–423.
Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008; 57(4): 1084–1092.
The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Acknowledgements
This research was supported by grants from the South-Eastern Regional Health Authorities and the Research Council of Norway. The EIRA study was supported by grants from: the Swedish Medical Research Council, the Swedish Council for Working Life and Social Research, King Gustaf V:s 80-year foundation, the Swedish Rheumatism Foundation, Stockholm County Council, the insurance company AFA, the EU-supported AutoCure project, NIH (P60 AR047782), and the COMBINE (Controlling chronic inflammatory diseases with combined efforts) project. We thank the Norwegian Bone Marrow Donor Registry for access to control materials, and would like to acknowledge Eva Jemsby, Gull-Britt Almgren and Julia Boström for sample management of the EIRA material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Genes and Immunity website
Supplementary information
Rights and permissions
About this article
Cite this article
Eike, M., Skinningsrud, B., Ronninger, M. et al. CIITA gene variants are associated with rheumatoid arthritis in Scandinavian populations. Genes Immun 13, 431–436 (2012). https://doi.org/10.1038/gene.2012.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2012.11
Keywords
This article is cited by
-
Association of CIITA (rs8048002) and CLEC2D (rs2114870) gene variants and type 1 diabetes mellitus
Journal of Diabetes & Metabolic Disorders (2024)
-
NOD-like receptors in autoimmune diseases
Acta Pharmacologica Sinica (2021)
-
HLA-DRB1 genes and the expression dynamics of HLA CIITA determine the susceptibility to T2DM
Immunogenetics (2021)
-
Association between the functional MHC2TA −168 A/G polymorphism and susceptibility to rheumatoid arthritis: a meta-analysis
Clinical Rheumatology (2016)
-
Variability in the CIITA gene interacts with HLA in multiple sclerosis
Genes & Immunity (2014)