Abstract
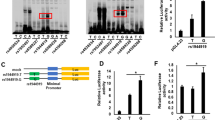
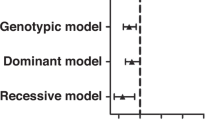
We fine mapped two primary biliary cirrhosis (PBC) risk loci, CLEC16A (C-type lectin domain family 16 member A)–suppressor of cytokine signaling 1 (SOCS1) and Spi-B protein (SPIB) and sequenced a locus, sialic acid acetylesterase (SIAE), proposed to harbor autoimmunity-associated mutations. In all, 1450 PBC cases and 2957 healthy controls were genotyped for 84 single-nucleotide polymorphisms (SNPs) across the CLEC16A-SOCS1 and SPIB loci. All 10 exons of the SIAE gene were resequenced in 381 cases and point substitutions of unknown significance assayed for activity and secretion. Fine mapping identified 26 SNPs across the CLEC16A-SOCS1 and 11 SNPs across the SPIB locus with significant association to PBC, the strongest signals at the CLEC16A-SOCS1 locus emanating from a SOCS1 intergenic SNP (rs243325; P=9.91 × 10−9) and at the SPIB locus from a SPIB intronic SNP (rs34944112; P=3.65 × 10−9). Among the associated SNPs at the CLEC16A-SOCS1 locus, two within the CLEC16A gene as well as one SOCS1 SNP (rs243325) remained significant after conditional logistic regression and contributed independently to risk. Sequencing of the SIAE gene and functional assays of newly identified variants revealed six patients with functional non-synonymous SIAE mutations (Fisher's P=9 × 10−4 vs controls) We demonstrate independent effects on risk of PBC for CLEC16A, SOCS1 and SPIB variants, while identifying functionally defective SIAE variants as potential factors in risk for PBC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ . Primary biliary cirrhosis. Hepatology 2009; 50: 291–308.
Hirschfield GM, Heathcote EJ, Gershwin ME . Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology 2010; 139: 1481–1496.
Hirschfield GM, Gershwin ME . Primary biliary cirrhosis: one disease with many faces. Isr Med Assoc J 2011; 13: 55–59.
Hirschfield GM, Invernizzi P . Progress in the genetics of primary biliary cirrhosis. Semin Liver Dis 2011; 31: 147–156.
Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Gu X et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med 2009; 360: 2544–2555.
Hirschfield GM, Liu X, Han Y, Gorlov IP, Lu Y, Xu C et al. Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat Genet 2010; 42: 655–657.
Liu X, Invernizzi P, Lu Y, Kosoy R, Bianchi I, Podda M et al. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet 2010; 42: 658–660.
Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY et al. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet 2011; 43: 329–332.
Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature 2007; 448: 591–594.
Palmer DC, Restifo NP . Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol 2009; 30: 592–602.
Ramanathan S, Dubois S, Gagnon J, Leblanc C, Mariathasan S, Ferbeyre G et al. Regulation of cytokine-driven functional differentiation of CD8T cells by suppressor of cytokine signaling 1 controls autoimmunity and preserves their proliferative capacity toward foreign antigens. J Immunol 2010; 185: 357–366.
Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet 2010; 42: 295–302.
Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 2007; 357: 851–862.
Ferreira RC, Pan-Hammarstrom Q, Graham RR, Gateva V, Fontan G, Lee AT et al. Association of IFIH1 and other autoimmunity risk alleles with selective IgA deficiency. Nat Genet 2010; 42: 777–780.
Zuvich RL, Bush WS, McCauley JL, Beecham AH, De Jager PL, Ivinson AJ et al. Interrogating the complex role of chromosome 16p13.13 in multiple sclerosis susceptibility: Independent genetic signals in the CIITA-CLEC16A-SOCS1 gene complex. Hum Mol Genet 2011; 20: 3517–3524.
Surolia I, Pirnie SP, Chellappa V, Taylor KN, Cariappa A, Moya J et al. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature 2010; 466: 243–247.
Cariappa A, Takematsu H, Liu H, Diaz S, Haider K, Boboila C et al. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J Exp Med 2009; 206: 125–138.
Szymanski K, Skorka A, Szypowska A, Bednarczuk T, Ploski R . Functionally defective germline variant of sialic acid acetylesterase (Met89Val) is not associated with type 1 diabetes mellitus and Graves’ disease in a Polish population. Tissue Antigens 2011; 78: 214–216.
Fujimoto M, Naka T . SOCS1, a negative regulator of cytokine signals and TLR responses, in human liver diseases. Gastroenterol Res Pract 2010 (doi:10.1155/2010/470468).
Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 1999; 98: 597–608.
Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 1999; 98: 609–616.
Torisu T, Nakaya M, Watanabe S, Hashimoto M, Yoshida H, Chinen T et al. Suppressor of cytokine signaling 1 protects mice against concanavalin A-induced hepatitis by inhibiting apoptosis. Hepatology 2008; 47: 1644–1654.
Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J Immunol 2008; 180: 3746–3756.
DeKoter RP, Geadah M, Khoosal S, Xu LS, Thillainadesan G, Torchia J et al. Regulation of follicular B cell differentiation by the related E26 transformation-specific transcription factors PU.1, Spi-B, and Spi-C. J Immunol 2010; 185: 7374–7384.
Nagasawa M, Schmidlin H, Hazekamp MG, Schotte R, Blom B . Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur J Immunol 2008; 38: 2389–2400.
Acknowledgements
This work was supported by grants from the Canadian Institutes for Health Research (MOP74621), the Ontario Research Fund (RE01-061) and the Canadian Primary Biliary Cirrhosis Society (GMH and KAS), the US National Institutes of Health (RO1 DK80670), the American Gastroenterological Association and the AJ and Sigismunda Palumbo Charitable Trust (KNL) and Alliance for Lupus Research, the Center for the Study of Inflammatory Bowel Disease at MGH and the NIH (AI 064930, AI 076505 and AR 058481) to SP KAS holds the Sherman Family Chair in Genomic Medicine and a Canada Research Chair award. We thank the NHLBI GO Exome Sequencing Project and its ongoing studies, which produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the WHI Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
Author contributions: GMH, GX and KAS designed the study. GX, EL, YS, VC, SP and CIA performed the genotyping, variant expression/functional analysis and data analysis. GMH, BDJ, CC, ALM, PM, RPM, JAO, VAL, BB, HB, VL, CV, CL, KNL and KAS developed the clinical network and sample collection and processing framework required for case and control accrual. GMH, GX and KAS wrote the manuscript. GMH, GX, SP, CIA and KAS vouch for the data and its analysis/interpretation. GMH and KAS decided in agreement with all other authors to publish this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Genes and Immunity website
Rights and permissions
About this article
Cite this article
Hirschfield, G., Xie, G., Lu, E. et al. Association of primary biliary cirrhosis with variants in the CLEC16A, SOCS1, SPIB and SIAE immunomodulatory genes. Genes Immun 13, 328–335 (2012). https://doi.org/10.1038/gene.2011.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2011.89
Keywords
This article is cited by
-
CLEC16A interacts with retromer and TRIM27, and its loss impairs endosomal trafficking and neurodevelopment
Human Genetics (2023)
-
Environmental factors, medical and family history, and comorbidities associated with primary biliary cholangitis in Japan: a multicenter case–control study
Journal of Gastroenterology (2022)
-
rs1944919 on chromosome 11q23.1 and its effector genes COLCA1/COLCA2 confer susceptibility to primary biliary cholangitis
Scientific Reports (2021)
-
The Genetic Contribution to Type 1 Diabetes
Current Diabetes Reports (2019)
-
POGLUT1, the putative effector gene driven by rs2293370 in primary biliary cholangitis susceptibility locus chromosome 3q13.33
Scientific Reports (2019)