Abstract
We previously found an association between faster CD4+ T-cell recovery in HIV-infected patients receiving combination antiretroviral therapy (cART) and interleukin-7 receptor-α (IL-7Rα) haplotype-2 in a predominantly Caucasian cohort. This study aims to determine whether this association was also significant in Africans. Patients were recruited from the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort (n=352). We used survival analysis and linear mixed modelling (LMM) to determine factors associated with CD4 T-cell recovery. Eight IL-7Rα single-nucleotide polymorphisms (SNPs) were genotyped in both Africans and Caucasians (n=57). Soluble (s)IL-7Rα levels were measured by ELISA. In UARTO, IL-7Rα haplotype-2 was associated with slower CD4 T-cell recovery following cART by using survival analysis (P=0.020) and no association was found with LMM (P=0.958). The tagging-SNP for IL-7Rα haplotype-2 (rs6897932) was associated with decreased sIL-7Rα (P<0.001). The haplotypes for the IL-7Rα were significantly different in Africans and Caucasians. Using IL-7Rα genotypes we found slower CD4 T-cell recovery in UARTO patients was still associated with rs6897932 (P=0.009) and rs3194051 was associated with faster CD4 T-cell recovery (P=0.006). Unlike Caucasians, we did not demonstrate a significant association between IL-7Rα haplotype 2 and faster CD4 T-cell recovery in Africans. The IL-7Rα SNPs associated with CD4 T-cell recovery following cART differ in African and Caucasian cohorts.
Similar content being viewed by others
Introduction
Immune reconstitution following suppressive combination antiretroviral therapy (cART) is heterogeneous, and although most patients experience a significant increase in CD4 T-cell counts, many patients fail to achieve counts above 500 cells μl−1 even with prolonged treatment.1, 2, 3 These patients remain at increased risk of non-AIDS-related illnesses and mortality despite years of suppressive cART.4, 5, 6 Clinical or genetic factors that may predict impaired CD4 T-cell recovery could potentially be used to identify patients who would benefit from earlier initiation of cART.
Interleukin-7 (IL-7) is a non-redundant cytokine, which is required for the generation of new T-cells from the thymus7 and for the survival of existing T-cells in circulation.8 IL-7 binds to the dimerized receptors of IL-7 receptor-α (IL-7Rα) (CD127) and the γ-chain receptor (CD132) to exert its effect. The IL-7Rα is also found in soluble (s) form, as sIL-7Rα in plasma, and is produced as a result of splicing exon-6 from the IL-7Rα gene mRNA.9 Four common haplotypes of the IL-7Rα gene have been described in Caucasians10 and found to be associated with differential levels of sIL-7Rα.11 In HIV-infected patients, although IL-7 levels are elevated, the expression of IL-7Rα on T-cells and signalling through the receptor (measured by STAT-5 phosphorylation) is impaired and only partially corrected with cART.12, 13 Previous studies have found that impaired IL-7 responsiveness is significantly associated with poor CD4 T-cell recovery following cART.14, 15
Various clinical factors have been associated with impaired CD4 T-cell reconstitution following cART, including lower CD4 T-cell counts at initiation of cART,16, 17, 18, 19 being older at cART initiation1, 17, 19, 20 and higher levels of immune activation both before and while on cART as measured by T-cell activation markers (such as human leukocyte antigen (HLA)-DR+CD38+ expression).21, 22, 23, 24 Multiple host genetic factors have also been found to influence CD4 T-cell recovery.25, 26, 27, 28, 29, 30, 31
We recently demonstrated by using a multivariable model that IL-7Rα haplotype-2 was a significant predictor of more rapid CD4 T-cell recovery following suppressive cART in an Australian-based largely Caucasian HIV-infected cohort.22 Consistent with observations in haplotype-2 carriers in HIV-uninfected cohorts,9, 11, 32, 33 we found that HIV-infected patients who were homozygous for haplotype-2 had significantly lower concentrations of sIL-7Rα compared with non-haplotype-2 carriers.22 We therefore proposed that the reduced sIL-7Rα levels in haplotype-2 carriers may increase the availability of IL-7 to bind to membrane-bound IL-7Rα on T-cells, leading to faster CD4 T-cell recovery.
Our main goal in this study was to determine whether a similar relationship could be detected between IL-7Rα haplotype-2 and faster CD4 T-cell recovery in an independent African cohort. In addition, we compared IL-7Rα genotypes from SNPs across the gene for their association with CD4 T-cell recovery and sIL-7Rα levels in African and Caucasian HIV-infected patients.
Results
Patient characteristics and IL-7Rα haplotype-2
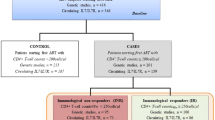
Patients receiving suppressive cART were selected from the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort. UARTO is a prospective observational cohort of HIV-infected patients receiving clinical care at the Immune Suppression Syndrome (ISS) Clinic in Mbarara who were recruited prior to cART initiation.34 Three hundred and fifty-nine patients fulfilled the inclusion criteria. Seven patients were excluded as they did not have baseline HIV RNA. The demographic and clinical details of these patients are shown in Table 1. The frequency of the SNP rs6897932 (C/T), which tags haplotype-2 in Caucasian patients, was found to be 9.6% in the UARTO cohort.
Association between IL-7Rα haplotype-2 and CD4 T-cell recovery following cART
To test whether IL-7Rα haplotype-2 was associated with shorter time to CD4>500 cells μl−1 following cART in the UARTO patients, we performed a multivariable Cox proportional hazard analysis, assessing haplotype association with the time taken to achieve the first of two consecutive CD4 T-cell counts >500 cells μl−1. This was the same analysis approach that we had previously used in our study of Caucasian patients.22 In the UARTO cohort, the median duration of follow up was 2.3 years (inter-quartile range 1.2–2.9). At 3 years, an estimated 6.4% (95% confidence interval (CI): 5.2–7.8%) of participants had achieved two successive CD4 T-cell counts >500 cells μl−1.
In unadjusted analyses, there was no association between participants carrying at least one copy of IL-7Rα haplotype-2 and time to two successive CD4 T-cell counts >500 cells μl−1 (hazards ratio: 0.55, 95% CI: 0.26–1.19, P=0.127). After adjustment for pre-treatment CD4 and CD8 T-cell count, baseline HIV RNA, age, gender and calendar year of cART initiation, carriage of IL-7Rα haplotype-2 as compared with non-haplotype-2 in UARTO was significantly associated with slower time to CD4 T-cell counts >500 cells μl−1 (AHR: 0.40, 95% CI: 0.18–0.87, P=0.020). Clinical factors that were found to be significantly associated with faster CD4 T-cell recovery in the UARTO patients were higher baseline CD4 T-cell counts (AHR: 2.93, 95% CI: 2.43–3.53, P<0.001), younger age at cART initiation (AHR: 0.94, 95% CI: 0.91–0.97, P<0.001) and cART initiation at an earlier calendar year (AHR: 0.74, 95% CI: 0.57–0.95, P=0.017) (Table 2).
We also used linear mixed-effects modelling (LMM) to assess CD4 T-cell recovery. Using LMM we found that greater increases in CD4 T-cells were significantly associated with higher baseline CD4 T-cell counts, younger age at cART initiation and earlier calendar year of cART initiation (data not shown); however, there was no evidence to suggest that haplotype-2 modified the slope of CD4 T-cell recovery after 3 months of cART in the multivariable model (β-coefficient: −0.03; 95% CI: −1.02–0.96, P=0.958) or the slope of early CD4 recovery (baseline–3 months) (β-coefficient=−0.54; 95% CI: −1.50–0.43, P=0.277).
IL-7Rα haplotypes are different in Africans and Caucasians
Given the lack of an association between IL-7Rα haplotype-2 and faster CD4 T-cell recovery in this study, which was in contrast to our prior findings in a Caucasian cohort,22 and given that Africans are known to have shorter and more varied haplotypes,35 we next determined whether other IL-7Rα SNPs or their haplotypes were associated with rate of recovery of CD4 T-cells in the UARTO cohort.
We first genotyped eight SNPs in the IL-7Rα gene in the UARTO cohort as well as in predominantly Caucasian HIV-infected patients recruited at the Alfred hospital in Melbourne, Australia (n=57). Two of these SNPs (rs11567685 and rs11567686) were in the promoter region, and were used in our original study,22 because they tagged haplotypes across the gene in Caucasians. One SNP was in exon-6 (rs6897932), the functionally significant SNP that regulates the splicing of exon-6 and tags haplotype-2. We also genotyped five additional coding region SNPs in the UARTO cohort (n=352) flanking the exon-6 SNP and potentially altering exon-6 splicing.
Unlike the Alfred patients, the SNPs in the promoter region were not in linkage disequilibrium (LD) with the SNPs flanking exon-6 in the UARTO patients. The SNPs in the coding region defined shortened haplotypes 1, 2 and 3 in both Alfred and UARTO patients (Figure 1). The minor allele at rs3194051, which tagged haplotype-4 in the Alfred patients, was split over three haplotypes in the UARTO patients. Therefore, the SNPs used to tag IL-7Rα haplotypes in Caucasians defined different haplotypes in Africans.
We next re-examined the association between IL-7Rα genotype (rather than the allele) and CD4 T-cell recovery. Using survival analysis, we confirmed our earlier findings that the rs6897932 SNP (CT versus CC) that tagged IL-7Rα haplotype-2 was associated with slower CD4 T-cell recovery (AHR: 0.33, 95% CI: 0.14–0.76, P=0.009). We also found that patients homozygous for the minor allele-G at rs3194051 were associated with faster CD4 T-cell recovery to 500 cells μl−1 (GG versus AA; AHR: 3.63, 95% CI: 1.45–9.07, P=0.006; Table 3). Neither of these SNPs were significantly associated with CD4 T-cell recovery by using LMM (data not shown).
Taken together, these data demonstrate that the difference in genotype and haplotypes for the IL-7Rα between Africans and Caucasians did not explain the different association of haplotype-2 with CD4 T-cell recovery in the two cohorts.
The distribution of the IL-7Rα genotypes and their association with sIL-7Rα levels in the UARTO and Alfred patients
Given the different relationship between the rs6897932 SNP (CT versus CC) that tagged IL-7Rα haplotype-2 and CD4 recovery in the UARTO and Alfred patients, we next compared the genotype distribution and the frequency of allele carriage between the UARTO and Alfred patients for the two promoter SNPs and the six IL-7Rα coding-region SNPs (Table 4). Allele frequencies were quite different in the UARTO cohort, and four minor alleles at loci rs11567762, rs1494555, rs6897932 and rs11567686 had significantly lower frequencies in UARTO as compared with the Alfred patients (P<0.001 for all).
The association between IL-7Rα genotype and sIL-7Rα levels were also assessed in the two cohorts. We found the SNP at rs6897932 in both cohorts and the SNP at rs987106 in the Alfred patients to be significantly associated with sIL-7Rα levels (Figures 2a and b). The T-allele at rs6897932 (which tags the IL-Rα haplotype-2) was associated with significantly lower levels of sIL-7Rα in both UARTO and Alfred patients (P<0.001, respectively), whereas the T-allele at rs987106 was associated with significantly higher sIL-7Rα levels in the Alfred patients (P=0.009) but not in the UARTO patients (P=0.107) (Figures 2c and d). None of the other IL-7Rα genotypes were associated with higher or lower sIL-7Rα levels (data not shown), including the rs3194051 SNP, which was associated with faster CD4 T-cell recovery in the UARTO patients (Figures 2e and f).
Association between three IL-7Rα SNPs (rs6897932, rs987106 and rs3194051) and sIL-7Rα levels in the Alfred and UARTO patients. Concentration of sIL-7Rα were square root-transformed and compared across the three genotypes for the SNP rs6897932 in (a) the Alfred and (b) UARTO patients; SNP rs987106 in the (c) Alfred and (d) UARTO patients; and SNP rs3194051 in the (e) Alfred and (f) UARTO patients. Comparisons across three genotypes were performed by using ANOVA* or t-test** when only two groups were available, followed by a post-hoc test (Dunnett's T3#). Only significant P-values are shown. Soluble IL-7Rα values were back transformed in the plots and the horizontal lines represent the mean sIL-7Rα concentration for each genotype.
Finally, we compared sIL-7Rα levels for a given IL-7Rα genotype between the UARTO and Alfred patients, and found that the mean sIL-7Rα concentration was not significantly different in the minor allele carriers in the two cohorts for all the eight SNPs tested when adjusted for CD4 T-cell counts (data not shown).
Discussion
We assessed if IL-7Rα haplotype-2 was associated with faster CD4 T-cell recovery following suppressive cART as we described previously in a cohort involving predominantly Caucasian HIV-infected patients. We were not able to demonstrate an association between IL-7Rα haplotype-2 and faster CD4 T-cell recovery following cART in this large observational cohort recruited in Uganda by using either survival analysis or LMM. In addition, although there were differences in the IL-7Rα haplotypes in the UARTO and Alfred patients, the tagging SNP that identified haplotype-2 (rs6897932), and also thought to be the functional SNP, was the same in both patient cohorts. Patients with T-allele at rs6897932 had significantly lower sIL-7Rα levels compared with non-T allele carriers, as we found in a predominantly Caucasian cohort.
We found that there was a greater degree of recombination in the IL-7Rα gene in UARTO patients as compared with Caucasians, as commonly reported in African populations.35 There were also significant differences in the population distribution of SNPs and haplotype (largely involving rs11567762, rs1494555, rs6897932 and rs11567686), consistent with the ethnic differences for these minor allele frequencies reported in other sub-Saharan African populations (Nigerians and Kenyans) in HapMap. This is the first report of the rs11567686 minor allele frequency in Africans.
Despite similar associations between soluble IL-7Rα and the IL-7Rα SNPs in the Alfred and UARTO patients, we did not find the same genetic association between the IL-7Rα SNP rs6897932, which tags haplotype-2, and the time to CD4 T-cells >500 cells μl−1 in the UARTO patients as described previously in Caucasian patients.22 The reason for this difference is currently unclear. Given the greater degree of recombination in the IL-7Rα gene seen in the UARTO patients as compared with Caucasians, it is possible that the rs6897932 SNP may be in LD with SNPs mediating other changes (not restricted to sIL-7Rα levels), which may have a different influence on CD4 T-cell recovery following cART in Africans. Another interpretation of the different findings in Caucasians and Africans could be that our initial findings in the Caucasian cohort may not have been related to levels of sIL-7Rα but could have been due to other SNPs in LD with rs6897932, which may have had an influence on CD4 T-cell recovery. This is of course possible but we believe this is less likely given the clear relationship in vitro between sIL-7Rα levels and IL-7 function in primary T-cells,36, 37 and that sIL-7Rα has a binding affinity similar to membrane-bound IL-7Rα on T-cells.38 Finding the opposite association between genotype and phenotype in replicate genetic studies has been described previously as a ‘flip-flop phenomenon,’39 and is commonly reported in genetic association studies across different ethnic groups.40, 41, 42 This may occur because of differences in LD between the variant genotyped and the true causal SNP, differences in haplotype frequencies or differences in the frequency of modifying genes that interact with the causal variant across the different populations studied.39
There may be other interpretations of our findings of different genetic associations in the UARTO and Caucasian patients in our prior study. First, there may have been significant differences in the immunological characteristics of the UARTO patients as compared with the Caucasian patients, which may have altered the relationship between the IL-7Rα genotype, sIL-7Rα levels and time to CD4 T-cell >500 cells μl−1 in the two cohorts, even though we used identical inclusion criteria. The patients in our previous study based in Australia were less immunosuppressed at cART initiation (median (inter-quartile range) baseline CD4 T-cell counts in Caucasians22 versus UARTO: 199 (92–304) versus 135 (77–201) cells μl−1) and had received suppressive cART for a longer duration (median (inter-quartile range) in Caucasians.22 versus UARTO: 4 (2.6–7.2) versus 2.3 (1.2–2.9) years). Second, recovery of CD4 T-cell subsets following cART may be persistently skewed in patients starting cART at low CD4 T-cell nadir with lower naïve and higher effector T-cell subsets compared with patients starting therapy at higher CD4 T-cell counts.43, 44 Given that IL-7Rα is expressed at higher levels on naïve T-cells than effector cells,12 a change in the relative numbers of these subsets in patients starting cART at a lower CD4 T-cell count could potentially lead to a reduced influence of sIL-7Rα and IL-7 in driving CD4 T-cell recovery. Third, immune activation (largely measured as increased expression of CD38+ and HLA-DR+ on T-cells) has also been shown to be inversely correlated with IL-7Rα expression on CD4 T-cells in HIV-infected patients,45, 46 and immune activation has a negative impact on CD4 T-cell recovery.47, 48, 49 Although we did not compare the immune activation levels in the UARTO and Caucasian patients, numerous studies have described a higher degree of immune activation both in HIV-uninfected50, 51, 52, 53, 54 and HIV-infected African patients53, 54, 55, 56 as compared with patients from high-income countries owing to differences in the environment52 and prevalence of other co-infections.51 The combined effects of poorer CD4 T-cell gain owing to increased T-cell activation and a reduced expression of IL-7Rα on T-cells may potentially negate any beneficial effects of increased IL-7 availability.
We found that homozygous carriers of the G-allele at rs3194051 experienced faster time to CD4 T-cell >500 cells μl−1 compared with homozygous carriers of the A-allele in the UARTO patients by using survival analysis, but there was no significant association by using LMM. There was no difference in the sIL-7Rα concentration in carriers of this SNP (Figure 2f). This SNP tags haplotype-4 in the Caucasian patients but not in the UARTO patients. In Caucasians, haplotype-4 has been associated with increased expression of IL-7Rα on T-cells and an increased percentage of CD31+ naïve T-cells in both patients with multiple sclerosis and controls.57 This haplotype has also been associated with reduced upregulation of IL-7Rα measured in whole blood in response to interferon-β stimulation as compared with non-haplotype-4 carriers.58 The relevance of these haplotype-4-associated immunophenotypes in influencing the time to CD4 T-cell >500 cells μl−1 in Africans carrying the minor G-allele at rs3194051 is currently unknown, but it is unlikely to be related to differences in the levels of sIL-7Rα.
We used two separate strategies to assess CD4 T-cell recovery—survival analysis and the more commonly used LMM.1, 59 We found that the identical clinical parameters were associated with increased rate of CD4 T-cell recovery by using both approaches and were consistent with other observational studies of CD4 T-cell recovery after cART.2, 16 It is important to note however, that this study was designed to replicate our previous study of Caucasians22 where we had previously used a survival analysis approach. However, in this study of the UARTO patients, there was a much lower frequency of patients achieving the event (that is, CD4 T-cells >500cells μl−1) as compared with the Caucasians in our prior study22 (15 versus 80% respectively) and this may have led to over-fitting of the Cox model. The advantage of LMM is that it measures the rate of CD4 T-cell recovery and is not affected by the limited number of patients achieving CD4 T-cells >500cells μl−1. Given our findings in the UARTO patients by using survival analysis and LMM, we went back to analyse the data from our previous Caucasian cohort22 by using LMM and confirmed that haplotype-2 carriers were still associated with faster CD4 T-cell recovery (data not shown). Although survival analysis and LMM identified different associations of haplotype-2 in the UARTO patients, neither approach identified a significant correlation with faster CD4 T-cell recovery as we had demonstrated previously in a predominantly Caucasian cohort.22
Two SNPs were associated with different concentrations of sIL-7Rα. In both the Alfred and UARTO cohorts, the T-allele at locus rs6897932 was associated with lower sIL-7Rα levels compared with the C-allele. This association has been shown previously in Caucasian cohorts,11, 22 and rs6897932 is thought to be the functional SNP altering the splicing and production of soluble IL7Rα.9, 32 Our findings are the first description of a significant association between sIL-7Rα levels and the SNP at rs6897932 in Africans.
The second association found in this study between rs987106 and soluble IL-7Rα levels has not been described previously in either population. The Alfred patients carrying the T-allele at this locus had significantly higher sIL-7Rα levels compared with patients carrying the A-allele. In prior case–control studies involving Nordic patients, carriers of the T-allele at this locus had an increased risk of multiple sclerosis,60, 61 although replicate studies of multiple sclerosis patients from the United States did not find a similar association.32 We did not find a relationship between the rs987106 SNP and sIL-7Rα levels in the UARTO patients. The lack of association however was not due to differences in the concentration of sIL-7Rα in T-allele (minor allele) carriers in the two groups, but rather due to a lower sIL-7Rα level in the A-allele (major allele) carriers in the Alfred patients as compared with the UARTO patients (Figures 2c and d). There were a higher percentage of A-allele carriers at rs987106 in the Alfred patients who also carried the T-alleles at rs6897932 as compared with the UARTO patients (53 versus 25%). Therefore, the reduced sIL-7Rα levels among the carriers of the A-allele at rs987106 in the Alfred patients might be an effect mainly driven by the co-carriage of the T-alleles at rs6897932, which would have had a smaller influence on the sIL7Rα levels measured in the A-allele carriers from the UARTO cohort. Therefore, it is still unclear if the rs987106 SNP is significantly associated with sIL-7Rα levels, and future studies in other cohorts will help confirm this.
We also compared the sIL-7Rα concentration in the minor allele carriers in both the Alfred and UARTO patients for all eight loci. After adjustments for differences in CD4 T-cell counts at sampling in the two cohorts, all SNPs were associated with similar sIL-7Rα levels. These data suggest that all the IL-7Rα SNPs tested in this study had similar effects on the sIL-7Rα levels in both Africans and Caucasians.
There were a few important limitations to our study. First, the duration of follow-up in the UARTO cohort was shorter than the follow-up duration in our prior study.22 As we do not know the exact mechanism of how sIL-7Rα influences CD4 T-cell recovery and if the effects on T-cell homeostasis may be apparent both in the early and late phases of CD4 T-cell recovery, we are uncertain how the difference in the duration of follow-up may have affected the results. Second, we were not able to assess markers of immune activation or the expression of IL-7Rα on T-cell subsets in the UARTO and Caucasian cohorts. Characterizing the immunological differences in the two cohorts would have provided some insight into the possible reasons for the difference in association between IL-7Rα haplotype-2 and time to CD4 T-cell >500 cells μl−1 in the two cohorts, and possibly a better understanding of the underlying mechanism for this association. Future work assessing the association between IL-7Rα haplotype-2 and CD4 T-cell recovery in HIV-infected patients should include an assessment of these immunological parameters.
In summary, we found that IL-7Rα haplotype-2 was not associated with faster CD4 T-cell recovery after cART in the UARTO cohort and this was contrary to our findings in Caucasians. The difference in association was not explained by a different relationship between IL-7Rα genotype and sIL-7Rα levels in the UARTO patients and Caucasians. Other factors that may potentially explain these findings include other genetic factors not tested in this study, differences in the degree of immunosuppression prior to cART or differences in environmental factors. The relationship between IL-7Rα haplotype-2 and CD4 T-cell recovery warrants further confirmation in an independent Caucasian cohort.
Materials and methods
Study population
Patients were recruited from the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort just prior to cART initiation and were observed at 3-monthly intervals in addition to their routine clinical care. HIV RNA level and CD4 T-cell count were assessed at each visit. At recruitment all patients provided written informed consent for plasma, buffy coat and saliva samples to be stored for future use, including genetic testing, and no additional ethical approval was required to perform this study.
Patient selection
From this cohort of 500 patients, we selected patients who fulfilled the following inclusion criteria: patients aged at least 18 years, first cART regimen consisting of combination cART (defined as at least three antiretroviral drugs), cART commenced at CD4 T-cell counts <500 cells μl−1 and patients achieved undetectable viral load (HIV RNA <400 copies per millilitre) by 6 months of cART initiation. Patients were excluded if they received a combination of didanosine and tenofovir as part of their cART regimen.
Demographic and clinical parameters such as cART regimen; date of antiretroviral initiation; and all CD4 T-cell, CD8 T-cell and HIV RNA measures from baseline to the most recent follow-up date were obtained from a centralized database. Observations of CD4 T-cell recovery were censored when patients showed evidence or were likely to have virological rebound (a single viral load >1000 copies per millilitre or two consecutive viral loads >400 copies per millilitre or confirmed treatment interruption of >2 weeks), or if their virological status was undetermined for a prolonged period (frequency of plasma viral load determinations reduced to <2 per year).
IL-7Rα genotyping and identification of the IL-7Rα haplotypes
DNA extraction was performed by using the DNA Blood and Tissue isolation kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions from cryopreserved buffy coats. The SNP that tagged haplotype-2 in Caucasians, as described previously10 and shown in Figure 1, was genotyped.
To compare differences between IL-7Rα genotypes in Africans and Caucasians, 57 patients from a previously described clinic-based cohort in Australia22 and the UARTO patients were further genotyped for six coding-region SNPs (rs1494555, rs6897932, rs3194051, rs11567762, rs987106 and rs3822731) and two promoter-region SNPs (rs11567685 and rs11567686). All genotyping was performed by using the Sequenom MassARRAY iPLEX platform at the Australian Genome Research Facility.
Genotyping data were analysed for LD by using HaploView 4.2(ref. 62) (Supplementary Figure 1). LD blocks were identified by using the confidence interval setting. The data from the HapMap populations were also examined for this gene (Supplementary Figure 2).
Determination of soluble IL-7Rα levels in plasma
Stored plasma samples obtained at any time point after at least 12 months of suppressive cART in the UARTO and Alfred patients were used to measured sIL-7Rα levels by using an ELISA-based assay as described previously.11 Each sample was tested in duplicate.
Statistical analysis
The association between the IL-7Rα haplotypes and CD4 T-cell recovery was assessed by using two methods: the Cox proportional hazard model was used to assess variables that were associated with the time taken to achieve a clinically relevant CD4 T-cell threshold after cART. The outcome was defined as the time taken to achieve CD4 T-cell counts >500 cells μl−1, where the period from baseline to the first of two consecutive time points with CD4 counts >500 cells μl−1 was considered the time to event. Candidate predictors were identified from univariable analysis (P-value <0.2) and included in the multivariable model where variables with a P-value of <0.05 were considered significant.
Piecewise regression by means of LMM using maximum likelihood estimation was also performed to assess the association between IL-7Rα haplotype-2 and the rate of CD4 T-cell increase at two different time periods (baseline to 3 months and >3 months) after cART initiation. As CD4 T-cell count was significantly skewed, it was square root-transformed to approximate normality. After confirming significantly different trends in the gradient of square root-transformed CD4 T-cell count before and after 3 months of cART, mixed-effects modelling was used to assess the association between clinical parameters and IL-7Rα haplotype/genotype with the CD4 T-cell gradient for each time period separately.
Differences between the distribution of the IL-7Rα genotypes in the UARTO and Alfred patients were assessed by using χ2/Fishers exact test. The association between the IL-7Rα genotypes and sIL-7Rα levels were tested by using analysis of variance (ANOVA) (with post-hoc Dunnett's T3 test)/t-test. A linear regression analysis was also used to adjust for differences in CD4 T-cell counts in the Alfred and UARTO patients when comparing the sIL-7Rα levels in the two groups. sIL-7Rα levels were square root-transformed for these analyses and a P-value <0.05 was considered significant. All analyses were performed by using Stata version 11.0 (StataCorp, College Station, TX, USA).
References
Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata MM et al. Incomplete peripheral CD4(+) cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009; 48: 787–794.
Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/[mu]L in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis 2005; 41: 361–372.
Rajasuriar R, Gouillou M, Spelman T, Read T, Hoy J, Law M et al. Clinical predictors of immune reconstitution following long-term combination antiretroviral therapy (cART) in patients from the Australian HIV observational database (AHOD). PLoS One 2011; 6: e20713.
Lichtenstein K, Armon C, Buchacz K, Chmiel J, Buckner K, Tedaldi EM et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010; 51: 435–447.
Prosperi MC, Cozzi-Lepri A, Castagna A, Mussini C, Murri R, Giacometti A et al. Incidence of malignancies in HIV-infected patients and prognostic role of current CD4 cell count: evidence from a large Italian cohort study. Clin Infect Dis 2010; 50: 1316–1321.
Lewden C, Chane G, Morlat P, Raffi F, Dupon M, Dellamonica P et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr 2007; 46: 72–77.
Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 1994; 180: 1955–1960.
Schluns KS, Kieper WC, Jameson SC, Lefrancois L . Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 2000; 1: 426–432.
Booth DR, Arthur AT, Teutsch SM, Bye C, Rubio J, Armati PJ et al. Gene expression and genotyping studies implicate the interleukin 7 receptor in the pathogenesis of primary progressive multiple sclerosis. J Mol Med 2005; 83: 822–830.
Teutsch SM, Booth DR, Bennetts BH, Heard RN, Stewart GJ . Identification of 11 novel and common single nucleotide polymorphisms in the interleukin-7 receptor-alpha gene and their associations with multiple sclerosis. Eur J Hum Genet 2003; 11: 509–515.
Hoe E, McKay FC, Schibeci SD, Gandhi K, Heard RN, Stewart G et al. Functionally significant differences in expression of disease-associated IL-7Rα haplotypes in CD 4 T cells and dendritic cells. J Immunol 2010; 184: 2512–2517.
Rethi B, Fluur C, Atlas A, Krzyzowska M, Mowafi F, Grutzmeier S et al. Loss of IL-7Ralpha is associated with CD4 T-cell depletion, high interleukin-7 levels and CD28 downregulation in HIV infected patients. AIDS 2005; 19: 2077–2086.
Juffroy O, Bugault F, Lambotte O, Landires I, Viard JP, Niel L et al. Dual mechanism of impairment of interleukin-7 (IL-7) responses in human immunodeficiency virus infection: decreased IL-7 binding and abnormal activation of the JAK/STAT5 pathway. J Virol 2010; 84: 96–108.
Colle J, Moreau J-L, Fontanet A, Lambotte O, Joussemet M, Jacod S et al. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients—effects of antiretroviral therapy. J Acquir Immune Defic Syndr 2006; 42: 277–285.
Camargo JF, Kulkarni H, Agan BK, Gaitan AA, Beachy LA, Srinivas S et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis 2009; 199: 1872–1882.
Kaufmann GR, Bloch M, Finlayson R, Zaunders J, Smith D, Cooper DA . The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. AIDS 2002; 16: 359–367.
Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med 2003; 163: 2187–2195.
Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr 2007; 45: 183–192.
Moore RD, Keruly JC . CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis 2007; 44: 441–446.
Althoff KN, Justice AC, Gange SJ, Deeks SG, Saag MS, Silverberg MJ et al. Virologic and immunologic response to HAART, by age and regimen class. AIDS 2010; 24: 2469–2479.
Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12: 1365–1371.
Rajasuriar R, Booth D, Solomon A, Chua K, Spelman T, Gouillou M et al. Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor α and microbial translocation. J Infect Dis 2010; 202: 1254–1264.
Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197: 126–133.
French MA, King MS, Tschampa JM, da Silva BA, Landay AL . Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009; 200: 1212–1215.
Nasi M, Pinti M, Bugarini R, Troiano L, Lugli E, Bellodi C et al. Genetic polymorphisms of Fas (CD95) and Fas ligand (CD178) influence the rise in CD4+ T cell count after antiretroviral therapy in drug-naive HIV-positive patients. Immunogenetics 2005; 57: 628–635.
Ahuja SK, Kulkarni H, Catano G, Agan BK, Camargo JF, He W et al. CCL3L1-CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV-1-infected individuals. Nat Med 2008; 14: 413–420.
O’Brien TR, McDermott DH, Ioannidis JPA, Carrington M, Murphy PM, Havlir DV et al. Effect of chemokine receptor gene polymorphisms on the response to potent antiretroviral therapy. AIDS 2000; 14: 821–826.
Puissant B, Roubinet F, Massip P, Sandres-Saune K, Apoil PA, Abbal M et al. Analysis of CCR5, CCR2, CX3CR1, and SDF1 polymorphisms in HIV-positive treated patients: impact on response to HAART and on peripheral T lymphocyte counts. AIDS Res Hum Retroviruses 2006; 22: 153–162.
Rigato PO, Hong MA, Casseb J, Ueda M, de Castro I, Benard G et al. Better CD4+ T cell recovery in Brazilian HIV-infected individuals under HAART due to cumulative carriage of SDF-1-3′A, CCR2-V64I, CCR5-D32 and CCR5-promoter 59029A/G polymorphisms. Curr HIV Res 2008; 6: 466–473.
Fernandez S, Rosenow AA, James IR, Roberts SG, Nolan RC, French MA et al. Recovery of CD4+ T cells in HIV patients with a stable virologic response to antiretroviral therapy is associated with polymorphisms of interleukin-6 and central major histocompatibility complex genes. J Acquir Immune Defic Syndr 2006; 41: 1–5.
Haas DW, Geraghty DE, Andersen J, Mar J, Motsinger AA, D’Aquila RT et al. Immunogenetics of CD4 lymphocyte count recovery during antiretroviral therapy: an AIDS Clinical Trials Group study. J Infect Dis 2006; 194: 1098–1107.
Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A et al. Interleukin 7 receptor [alpha] chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet 2007; 39: 1083–1091.
McKay FC, Swain LI, Schibeci SD, Rubio JP, Kilpatrick TJ, Heard RN et al. Haplotypes of the interleukin 7 receptor alpha gene are correlated with altered expression in whole blood cells in multiple sclerosis. Genes Immun 2007; 9: 1–6.
Bousheri S, Burke C, Ssewanyana I, Harrigan R, Martin J, Hunt P et al. Infection with different hiv subtypes is associated with CD4 activation-associated dysfunction and apoptosis. J Acquir Immune Defic Syndr 2009; 52: 548–552.
Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B et al. The structure of haplotype blocks in the human genome. Science 2002; 296: 2225–2229.
Crawley AM, Faucher S, Angel JB . Soluble IL-7Ralpha (sCD127) inhibits IL-7 activity and is increased in HIV infection. J Immunol 2010; 184: 4679–4687.
Hartgring SA, van Roon JA, Wenting-van Wijk M, Jacobs KM, Jahangier ZN, Willis CR et al. Elevated expression of interleukin-7 receptor in inflamed joints mediates interleukin-7-induced immune activation in rheumatoid arthritis. Arthritis Rheum 2009; 60: 2595–2605.
Rose T, Lambotte O, Pallier C, Delfraissy JF, Colle JH . Identification and biochemical characterization of human plasma soluble IL-7R: lower concentrations in HIV-1-infected patients. J Immunol 2009; 182: 7389–7397.
Lin PI, Vance JM, Pericak-Vance MA, Martin ER . No gene is an island: the flip-flop phenomenon. Am J Hum Genet 2007; 80: 531–538.
Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN . Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 2003; 33: 177–182.
Tan EK, Tan C, Shen H, Chai A, Lum SY, Teoh ML et al. Alpha synuclein promoter and risk of Parkinson's disease: microsatellite and allelic size variability. Neurosci Lett 2003; 336: 70–72.
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J et al. Alpha-synuclein locus triplication causes Parkinson's disease. Science 2003; 302: 841.
Robbins GK, Spritzler JG, Chan ES, Asmuth DM, Gandhi RT, Rodriguez BA et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS clinical trials group protocol 384. Clin Infect Dis 2009; 48: 350–361.
Sakai K, Gatanaga H, Takata H, Oka S, Takiguchi M . Comparison of CD4+ T-cell subset distribution in chronically infected HIV+ patients with various CD4 nadir counts. Microbes Infect 2010; 12: 374–381.
Koesters SA, Alimonti JB, Wachihi C, Matu L, Anzala O, Kimani J et al. IL-7Ralpha expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur J Immunol 2006; 36: 336–344.
Benito J, Lopez M, Lozano S, Gozalez-Lahoz J, Soriano V . Downregulation of interleukin-7 receptor (CD127) in HIV infection is associated with T cell activation and is a main factor influencing restoration of CD4(+) cells after antiretroviral therapy. J Infect Dis 2008; 198: 1466–1473.
Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187: 1534–1543.
Solomon A, Cameron PU, Bailey M, Dunne AL, Crowe SM, Hoy JF et al. Immunological and virological failure after antiretroviral therapy is associated with enhanced peripheral and thymic pathogenicity. J Infect Dis 2003; 187: 1915–1923.
Nakanjako D, Ssewanyana I, Mayanja-Kizza H, Kiragga A, Colebunders R, Manabe YC et al. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis 2011; 11: 43.
Koesters SA, Matu L, Kiama P, Anzala O, Embree J, Plummer FA et al. Elevation of immune activation in Kenyan women is associated with alterations in immune function: implications for vaccine development. J Clin Immunol 2004; 24: 702–709.
Borkow G, Leng Q, Weisman Z, Stein M, Galai N, Kalinkovich A et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest 2000; 106: 1053–1060.
Clerici M, Butto S, Lukwiya M, Saresella M, Declich S, Trabattoni D et al. Immune activation in Africa is environmentally-driven and is associated with upregulation of CCR5. Italian–Ugandan AIDS Project. AIDS 2000; 14: 2083–2092.
Lukwiya M, Rizzardini G, Trabattoni D, Piconi S, Saresella M, Declich S et al. Evaluation of immune activation in HIV-infected and uninfected African individuals by single-cell analysis of cytokine production. J Acquir Immune Defic Syndr 2001; 28: 429–436.
Rizzardini G, Trabattoni D, Saresella M, Piconi S, Lukwiya M, Declich S et al. Immune activation in HIV-infected African individuals. Italian–Ugandan AIDS cooperation program. AIDS 1998; 12: 2387–2396.
Hunt P, Martin J, Ssewanyana I, Bennett J, Emenyonu N, Kembabazi A et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. 17th Conference on Retroviruses & Opportunistic Infections, San Francisco, 16–19 Feb 2010: paper 306.
Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med 2000; 6: 1036–1042.
Broux B, Hellings N, Venken K, Rummens JL, Hensen K, Van Wijmeersch B et al. Haplotype 4 of the multiple sclerosis-associated interleukin-7 receptor alpha gene influences the frequency of recent thymic emigrants. Genes Immun 2010; 11: 326–333.
Hoe E, McKay F, Schibeci S, Heard R, Stewart G, Booth D . Interleukin 7 receptor alpha chain haplotypes vary in their influence on multiple sclerosis susceptibility and response to interferon Beta. J Interferon Cytokine Res 2010; 30: 291–298.
Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS 2003; 17: 1907–1915.
Lundmark F, Duvefelt K, Iacobaeus E, Kockum I, Wallstrom E, Khademi M et al. Variation in interleukin 7 receptor [alpha] chain (IL7R) influences risk of multiple sclerosis. Nat Genet 2007; 39: 1108–1113.
Zhang Z, Duvefelt K, Svensson F, Masterman T, Jonasdottir G, Salter H et al. Two genes encoding immune-regulatory molecules (LAG3 and IL7R) confer susceptibility to multiple sclerosis. Genes Immun 2005; 6: 145–152.
Barrett JC, Fry B, Maller J, Daly MJ . Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265.
Acknowledgements
RR is a recipient of the Kings Scholarship from the Malaysian Government and acknowledges the University of Malaya and the Ministry of Higher Education. SRL is an Australian National Health and Medical Research Council (NHMRC) practitioner fellow. SRL receives funding from the NHMRC and the Alfred Foundation. The UARTO cohort was funded by MH54907 and the Mark and Lisa Schwartz Family Foundation. DRB received additional funding from MH K-24 87227.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Genes and Immunity website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Rajasuriar, R., Booth, D., Gouillou, M. et al. The role of SNPs in the α-chain of the IL-7R gene in CD4+ T-cell recovery in HIV-infected African patients receiving suppressive cART. Genes Immun 13, 83–93 (2012). https://doi.org/10.1038/gene.2011.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2011.65
Keywords
This article is cited by
-
IL-7/IL7R axis dysfunction in adults with severe community-acquired pneumonia (CAP): a cross-sectional study
Scientific Reports (2022)
-
IL7RA genetic variants differentially affect IL-7Rα expression and alternative splicing: a role in autoimmune and infectious diseases?
Genes & Immunity (2020)
-
An IL7RA exon 5 polymorphism is associated with impaired IL-7Rα splicing and protection against tuberculosis in Ghana
Genes & Immunity (2019)
-
IL-7/IL-7R gene variants impact circulating IL-7/IL-7R homeostasis and ART-associated immune recovery status
Scientific Reports (2019)
-
Association between IL7R polymorphisms and severe liver disease in HIV/HCV coinfected patients: a cross-sectional study
Journal of Translational Medicine (2015)