Abstract
Purpose
To evaluate long-term structural and functional changes to the retina and optic nerve following panretinal photocoagulation (PRP) in diabetic retinopathy (DR) patients.
Methods
Participants were patients with DR requiring PRP and control patients with DR not requiring PRP. The Heidelberg retinal tomography (HRT) and optical coherence tomography (OCT) were performed to analyze the optic nerve and macula. Best-corrected visual acuity (BCVA) and visual field (VF) testing were done to measure central and peripheral vision. Wide-field fluorescein angiogram was performed to monitor the progression of diabetic ischemia. The primary outcome measure was to determine the degree of retinal and optic nerve changes before and after PRP.
Results
There was a non-significant thickening of the macula and retinal nerve fiber layer at 6 months post laser that recovered by 24 months. Mean perfused ratio was significantly increased (P=0.02) at 12 and 24 months post laser. Independently grading patient stereophotographs, grader 1 indicated there was a non-significant increase in cup to disk ratio post laser, while grader 2 indicated a significant increase at 6 (P=0.04), 12 (P=0.02), and 24 months (P=0.005). There was a significant VF decrease (P≤0.02) at 12 and 24 months post laser with BCVA showing a non-significant trend of deteriorating results.
Conclusion
Despite an improvement in peripheral perfusion, there was a significant progressive decline of peripheral VF over the study period. Clinical grading of the optic nerve was more unreliable following PRP, despite the absence of significant morphological changes as detected by the OCT and HRT.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is the leading cause of blindness among working-aged adults and is estimated to affect >141 million adults worldwide.1, 2 Over the past 40 years, epidemiological studies and clinical trials have demonstrated that timely laser photocoagulation can prevent vision loss in DR. Panretinal photocoagulation (PRP) has been shown to stabilize and control the proliferative disease in Diabetic Retinopathy Study and has since became the gold standard treatment.3, 4 While it has been reported that PRP may result in visual field changes, there have been few studies that have looked at the impact on the optic nerve, especially the long-term effects.5, 6, 7 The risk of a dual diagnosis of treated DR and normal-tension glaucoma exists without adequate awareness of the potential impact of PRP on the appearance of the optic nerve head. While PRP has revolutionized how DR patients are managed, the long-term structural and functional effects of PRP on the retina and optic nerve remain unanswered.
The purpose of this study is to evaluate retinal and optic nerve changes using structural and functional diagnostic tests at 6, 12, and 24 months in DR patients post laser treatment. To our knowledge, this is the first comprehensive study to investigate long-term structural and functional effects of PRP on the retina and optic nerve that involves analysis of macular thickness, RNFL, mean perfusion, vertical C/D ratio, stereophotos, IOP, visual acuity, and visual fields.
Materials and methods
The study was conducted at Ivey Eye Institute, a tertiary care academic center in London, Ontario, Canada. This study complies with Declaration of Helsinki and was approved by Health Sciences Research Ethics Board of Western University. Prospective, treatment-naive patients who had severe non-proliferative and proliferative DR according to the ETDRS guidelines and over the age of 18 years were recruited into the study. Participants were excluded from the study if they had advanced lens opacity or cataract that could affect diagnostic testing, history of glaucoma, or any other disorder of the optic nerve (optic neuropathy, neuritis, uveitis, or retinal degeneration), presence of macular edema (central macular thickness >300 μm), and prior retinal treatment (PRP, focal laser or surgery within 6 months of participation). All participants provided informed consent. From this group, eight patients who did not receive laser treatments as the DR was at the severe non-proliferative state were chosen as the control. Participants were evaluated at pre-laser baseline, and 6, 12, and 24 months post laser. During each study visit, participants underwent OCT, HRT, VF, and IVFA testing, as well as a standard ophthalmic exam that included best-corrected Snellen visual acuity, intraocular measurement using Goldmann applanation tonometer, and slip lamp examination. Humphrey 24-2 SITA visual field testing was performed with undilated eyes.
Optical coherence tomography
Macular thickness, optic disc cube and HD 5 line raster imaging was obtained using optical coherence tomography (Cirrus HD-OCT 4000, Carl Zeiss Meditech, Dublin, CA, USA). The principles and protocol have been previously described in detail.8, 9, 10 OCT tests were taken at pre-laser baseline, and at 6, 12, and 24 months post laser. Each scan was repeated three times to ensure highest signal strength.
Analysis of the macula were performed using 6 × 6 mm scans with a resolution of 512 × 128 (128 horizontal scan lines consisting of 512 A-scans). The macular thickness was displayed as three concentric circles, with a 1 mm diameter central circular subfield, a 3 mm diameter inner ring, and a 6 mm diameter outer ring. Each ring was divided into superior, inferior, nasal, and temporal quadrants.7 When performing a scan of the optic nerve, a 3.4 mm ring was centered on the optic nerve and 768 A-scans are performed.11, 12 The average RNFL thickness and vertical C/D ratio were measured. To analyze images of the retinal structure, HD 5 line raster was performed. This high-density scan does 4096 A-scans in five planes to provide a high-definition image.12
Heidelberg retinal tomography
HRT imaging of the optic nerve was performed by Heidelberg Retina Tomograph II. The scanning protocol has been previously described in detail.13, 14 Each scan was repeated three times to ensure lowest SD. In brief, an experienced technician marks the optic disc margin contour line (marked at the inner border of the scleral ring) by observing the disk images. Once the contour line is marked, a standard reference plane is placed 50 μm posterior to the mean retinal height between 350 and 356° along the contour line.15 The area superficial to the reference plane is considered the neuroretinal rim, while the area deep to the plane is considered the cup. HRT software analyzes the shape of the optic nerve, to either be normal or outside normal limits, by calculating a set of stereometric parameters. These parameters are also used to calculate glaucomatous progression.16
Humphrey visual field analyzer
Standard Humphrey 24-2 SITA testing algorithm was performed on Humphrey Field Analyzer, model 750i (Zeiss Humphrey Systems, Dublin, CA, USA). In 24-2 SITA testing algorithm, 54 individual points are tested. The threshold values calculated from those points are then compared to a normative database for similar aged normal-sighted individuals.17 Fixation losses and false-negative responses >20% and false-positive responses >15% were excluded from the study.16
Wide-field fluorescein angiogram and quantification of ischemia
Angiography was performed on the Optos 200 Tx with an intravenous injection of 5 ml of 10% sodium fluorescein.18 Digital stereoscopic photographs and fluorescein angiography (IVFA) photos were taken. The best IVFA image obtained during the arteriovenous phase of each patient was used to quantify the amount of ischemia. The uncompressed tiff images were transferred from the Optos V2 Vantage Review Software to Adobe software (Adobe Systems, Inc., San Jose, CA, USA) for analysis.
Two graders quantified the amount of ischemia in each patient using the concentric rings method that has been previously described in detail.19 In this method, the innermost circle was first centered on the optic nerve to be sized proportionately and then repositioned to be fovea-centered. The 12 segments in each of the rings were graded as, ‘perfused’, or ‘non-perfused’, if >50% of the segment showed either a perfused or non-perfused morphology. The classification of ‘non-perfused’ and ‘perfused’ morphology was taken from the SCORE study. In the control and PRP laser eyes, ‘non-perfused’ was characterized by a darker appearance of the retina and pruned tree appearance (narrowed and difficult to see) of surrounding arterioles. ‘Perfused’ morphology was characterized by its clear ground-glass opacity.20 Calculations of the mean perfused ratio were performed using the mean perfusion ratio=area perfused/(area perfused+non-perfused). Each segment in each of the rings was given a value of either 0 indicating non-perfused or 1 indicating perfused retina.
Optic nerve grading
Stereoscopic photographs were taken with Optos 200 Tx at baseline, and 6, 12, and 24 months post laser. Optic nerve photographs were cropped to a size of 4 × 4 mm for grading. Two glaucoma specialists independently graded the C/D ratio of the laser-treated eyes and control eyes of diabetic patients not requiring laser. The graders were masked to the type of patients and purpose of the study.
Pattern scan laser photocoagulator
Pascal (pattern scan laser photocoagulator) laser is a semi-automated 532 nm frequency-doubled neodymium-doped yttrium aluminum garnet (Nd:YAG) solid-state laser.21 The laser was applied in a pattern array and uses very short pulse durations of 10–20 ms for each burn with typically a higher power compared to conventional photocoagulation (100 mW and 100 ms pulse duration). All patients received PRP with a pulse duration of 20 ms and a spot size of 200 mm. Power varied between 430–600 mW to achieve a mild gray-white burn intensity, which according to the ETDRS guidelines, is a grade 2+ and 3+ burn. Patients received a mean±SD of 1720.8±392.3 laser spots ranging from 1125 to 2125 spots. Between sessions, there was an interval of 2 to 3 weeks.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). For statistical analysis, Snellen BCVA measurements were converted to logarithm of the minimum angle of resolution (logMAR) scores. Unpaired t-test was used to compare the average age, and Fisher’s exact test was used to compare the percentage of female participants between control and PRP laser groups. Paired t-test was used to compare differences at specified points over time in PRP-treated and control groups. Repeated measures ANOVA with Bonferroni’s multiple comparison test was used to statistically analyze the interval changes of measurements in PRP laser and control groups at baseline, 6, 12, and 24 months, respectively. All data were expressed as mean±SD and accepted as statistically significant if P<0.05.
To calculate sample size with an overall power of 80%, P<0.05 was used as an acceptable significance level. Using a two-tailed test for a control with an optic nerve of 0.3 and an atrophy group of 0.6, the effect size would be 0.3. Adding the values to the sample size calculation, we calculated that we would need 16 patients for an 80% power number. Twenty diabetic patients requiring PRP were recruited into the study, but four were removed due to patient compliance. Eight patients were used as diabetic controls not requiring laser. The diabetic control group did not have proliferative disease that developed during the 2-year study period. They were included to ensure that the observed changes could be correlated with the laser treatments and not due to diabetic disease itself.
Results
A single eye from each of the 16 patients requiring PRP and 8 control patients not requiring PRP, who were recruited between 20 December 2013 and 27 December 2015, was analyzed. The average age of patients was 70.6±8.4 years for the control group and 65.7±12.5 years for the PRP laser group. The control group had 37.5% female participants, while the PRP laser group had 25% female participants. The control and PRP laser groups did not significantly differ (P>0.05) in any of these categories. None of the patients developed diabetic macular edema during the study.
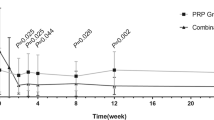
Following laser treatment, the macular thickness (Figure 1a) increased by 3.9 μM at 6 months (298.10±29.02 μM, P=0.44) relative to baseline (294.20±24.20 μM). The macular thickness recovered to 293.30±20.99 μM at 12 months (P=0.82) and 291.90±25.41 μM at 24 months (P=0.74). Compared to baseline (276.60±35.36 μM), the control group showed no significant change in macular thickness at 6 months (273.40±29.22 μM, P=0.27), 12 months (274.90±33.48 μM, P=0.72), and 24 months (272.80±36.51 μM, P=0.31).
Interval changes in (a) macular thickness, (b) RNFL thickness and (c) mean perfused ratio at baseline, and 6, 12, and 24 months later in the control and PRP laser groups. P-value: control group differences between baseline and post-follow-up measurements, paired t-test. P'-value: PRP laser group differences between baseline and post-laser measurements, paired t-test.
RNFL thickness (Figure 1b) increased at 6 months (86.25±12.07 μM, P=0.14), 12 months (85.50±9.70 μM, P=0.29), and 24 months (84.38±13.09 μM, P=0.56) compared to baseline (82.75±11.38 μM). The control group had no significant change in RNFL thickness at 6 months (74.88±9.96 μM, P=0.51), 12 months (74.38±8.78 μM, P=0.93), and 24 months (72.75±9.15 μM, P=0.40) compared to baseline (74.25±10.39 μM).
The mean perfused ratio showed no significant difference at 6 months (0.45±0.15, P=0.16), but a significant improvement at 12 months (0.47±0.14, P=0.02) and 24 months (0.49±0.15, P=0.02) compared to baseline (0.44±0.15) (Figure 1c). There was no significant difference in the control group at 6 months (0.61±0.21, P=1.00), 12 months (0.61±0.22, P=0.68), and 24 months (0.61±0.22, P=0.80) compared to baseline (0.61±0.21).
Analysis of the optic nerve and its vertical C/D ratio by OCT (Figure 2a) showed a slight increase at 6 months (0.48±0.19, P=0.43), 12 months (0.47±0.20, P=0.61), and 24 months (0.47±0.19, P=0.0.62) compared to baseline (0.46±0.19). Vertical C/D ratio remained consistent in the control group, being 0.52±0.29 at baseline, 0.54±0.23 at 6 months (P=0.57), 0.54±0.24 at 12 months (P=0.64), and 0.54±0.27at 24 months (P=0.37).
Interval changes in vertical cup/disk ratio measured on the (a) OCT and (b) HRT at baseline, 6, 12 and 24 months later in the control and PRP laser groups. P-value: control group differences between baseline and post-follow-up measurements, paired t-test. P'-value: PRP laser group differences between baseline and post-laser measurements, paired t-test.
Analysis of the optic nerve and its vertical C/D ratio using the HRT (Figure 2b) in the PRP laser group increased at 6 months (0.33±0.30, P=0.24) compared to baseline (0.27±0.26) measured. It remained elevated at 12 months (0.32±0.28, P=0.34) and 24 months (0.31±0.28, P=0.44) post-laser. Vertical C/D ratio showed no significant difference in the control group at 6 months (0.34±0.31, P=0.61), 12 months (0.32±0.30, P=0.44), and 24 months (0.33±0.29, P=0.51) compared to baseline (0.35±0.28).
Two glaucoma specialists were assigned to independently grade the vertical C/D ratio in the same eyes following PRP laser using stereophotographs (Figure 3a). Grader 1 indicated at baseline, the mean vertical C/D was 0.44±0.17, and noted an increase at 6 months (0.46±0.17, P=0.08), 12 months (0.46±0.16, P=0.15), and 24 months (0.47±0.16, P=0.13) post laser. Grader 2 indicated baseline mean vertical C/D ratio of 0.35±0.17, and noted a significant increase at 6 months (0.37±0.18, P=0.04) and 12 months (0.38±0.18, P=0.02), with a further increase at 24 months (0.40±0.19, P=0.005) post laser. The graders also graded control eyes of diabetic patients not requiring laser treatment. Both graders 1 and 2 found that over 24 months, there was no significant difference (P≥0.10; Figure 3b).
Interval changes in vertical cup/disk ratio measured by two masked glaucoma specialists using stereophotographs at baseline, 6, 12, and 24 months later in the (a) PRP laser group and the (b) control group. P-value: grader 1 differences between baseline and post-laser measurements, paired t-test. P'-value: grader 2 differences between baseline and post-laser measurements, paired t-test.
After the laser treatment, visual field tests had an average baseline mean deviation (MD) (Figure 4a) in the PRP laser group of −4.39±3.10 dB. Mean MD digressed at 6 months by 1.30 dB (−5.68±5.03 dB, P=0.24), and further decreased at 12 months (−5.73±4.07 dB, P=0.02) and 24 months (−6.36±3.74, P=0.01) post laser. Control group showed no significant differences in visual field mean MD at baseline (−8.46±8.83 dB), 6 months (−8.43±6.29 dB, P=0.98), 12 months (−7.41±4.78 dB, P=0.53), and 24 months (−9.19±6.35 dB, P=0.64).
Interval changes in mean deviation measured on the (a) Humphrey Visual Field Analyzer and (b) visual acuity at baseline, and 6, 12, and 24 months later in the control and PRP laser groups. P-value: control group differences between baseline and post-follow-up measurements, paired t-test. P'-value: PRP laser group differences between baseline and post-laser measurements, paired t-test.
Baseline mean best-corrected visual acuities (Figure 4b) were 0.32±0.24 logMAR in the control group and 0.34±0.31 logMAR in the PRP laser group. Visual acuity slightly increased to 0.3±0.29 logMAR at 6 months (P=0.32), but gradually decreased to 0.36±0.33 logMAR at 12 months (P=0.58) and 0.38±0.30 logMAR at 24 months (P=0.37) post laser. Visual acuity remained consistent in the control group, being 0.32±0.24 logMAR at baseline, 0.29±0.23 logMAR at 6 months (P=0.59), 0.31±0.21 logMAR at 12 months (P=0.90), and 0.33±0.20 logMAR at 24 months (P=0.85). These changes were not statistically significant.
After Pascal photocoagulation, intraocular pressure (IOP) (Figure 5) significantly decreased (P=0.04) by 1.5 mm Hg at 6 months (17.31±3.26 mm Hg) compared to baseline (18.81±4.04 mm Hg). It remained at 17.31±3.05 mm Hg at 12 months (P=0.12), but then increased close to control values (18.25±3.98 mm Hg) at 24 months (P=0.68). In the control group, IOP remained constant, being 17.38±2.00 mm Hg at baseline, 17.13±2.59 mm Hg at 6 months (P=0.81), 17.00±1.69 mm Hg at 12 months (P=0.70), and 17.50±3.30 mm Hg at 24 months (P=0.93). These changes were not statistically significant.
Interval changes in intraocular pressure at baseline, 6, 12, and 24 months later in the control and PRP laser groups. P-value: control group differences between baseline and post-follow-up measurements, paired t-test. P'-value: PRP laser group differences between baseline and post-laser measurements, paired t-test.
Discussion
PRP laser used in this study was Pascal Pattern Scan Laser (OptiMedica, Santa Clara, CA, USA). Compared to conventional photocoagulation lasers that use lower power (100 mW) and longer pulse duration (100 ms), Pascal laser that is applied in a pattern array, uses a very short pulse duration of 10–20 ms for each burn and typically a higher power. It has been suggested that Pascal laser may promote healing while causing less collateral damage compared to conventional photocoagulation lasers.22 Despite differences between Pascal laser and conventional argon laser for PRP, past studies have shown that there are no significant differences in outcomes between the two lasers, showing similar effects and similar regression of DR.22, 23, 24, 25
The macular and RNFL thickness (Figures 1a and b) showed an increase at 6 months and a decrease by 24 months post laser indicating recovery. Past studies7, 26, 27 have described similar increases in thickness pre-6 months, likely due to post-laser intraretinal inflammation. Photocoagulation creates thermal and photochemical damage, resulting in the production of reactive oxygen species (ROS).27 ROS generates an immune response in the retinal tissue by increasing the intraretinal junctional permeability and recruitment of leukocytes.7, 27, 28, 29, 30, 31 The decrease post 6 months suggests that the effects of an acute inflammation persist up to 6 months in the retinal tissue after laser treatment. Overall, by 24 months, there is no significant change in the macular or RNFL thickness compared to pre-treatment state.
To determine whether the observed changes were due to the laser treatment or diabetes itself, the amount of ischemia was quantified using the mean perfused ratio. The mean perfused ratio was calculated by the concentric rings method utilized by Nicholson et al.19 Compared to the standard ischemic index method wherein the grader draws the ratio of area perfused to the total area graded, the concentric rings method utilizes a set template. It has a perfect agreement with the ischemic index method, a higher inter-grader agreement, and a short-grading time.19 Using the concentric rings method, it was observed that at 12 and 24 months post laser, PRP laser resulted in a significant increase in the perfusion of the eye, whereas the control eyes showed no change. The result suggests that although diabetes remains a contributing factor, the improvement and changes in ischemia are most likely the result of the laser treatment.
There was a similar trend between the OCT and HRT machines when analyzing the optic nerve. In the vertical C/D ratio on OCT, there was a slight increase of +0.009 at 6, 12, and 24 months compared to the baseline in the PRP laser group. HRT showed an increase in the vertical C/D ratio by +0.06 post 6 months. There was a statistically significant decrease in pressure by 1.5 mm Hg at 6 months compared to baseline (18.81±4.04 mm Hg; Figure 5).32 IOP remained lowered before recovering to 18.25±3.98 mm Hg at 24 months.
The current gold standard for analyzing the optic nerve is grading of stereophotographs by glaucoma specialists. Two glaucoma specialists were assigned to grade the C/D ratio in the same eyes following PRP laser treatment using stereophotographs. Similarly to the OCT and HRT results, grader 1 indicated that there was a non-significant C/D increase over 24 months, whereas grader 2 indicated a significant increase at 6 and 12 months, with a further significant increase at 24 months post laser. There is a discrepancy between how ophthalmologists grade optic nerves post laser treatment. This has been observed in other studies of disc analysis and it is apparent that a more objective analysis is needed.33, 34 To verify whether the discrepancy is due to the laser-treated eyes and not the graders themselves, the graders also assessed control eyes of diabetic patients not requiring laser treatment. When asked to grade the control eyes, both graders 1 and 2 found that over 24 months, there was no significant difference in the C/D ratios. These data may suggest that non-morphological changes in color in the optic nerve post-PRP result in a decreased ability to consistently grade nerves for cupping that is important in recognizing the potential for hidden glaucoma.
In addition to the inconsistent clinical grading of the optic nerve post laser, visual acuity showed a non-significant decline, and visual fields showed a progressive significant decline at 12 and 24 months post laser. Past studies have reported the degree of peripheral retina destroyed by photocoagulation; however, none have documented progressive long-term visual field loss over a 2-year time period.5, 35, 36 With the unreliable grading of the optic nerve and further progressive deterioration of visual fields post laser, it is understandable why some diabetic patients post PRP are being misdiagnosed with having normal-tension glaucoma despite the presented symptoms that are likely attributed to iatrogenic effects of the laser.
One limitation of this study is that despite meeting power number calculations for the treatment group, 16 control patients in the study would have been preferred. Although the number of participants for the control group did not meet the limit, the control group did not show any significant difference in any of the testing and showed stable trends. As both glaucoma and the effects of PRP on RNFL and optic disk are often progressive, it would be enticing to extend the follow-up period beyond 2 years to increase the ability to discern small, yet progressive changes. It would be interesting to determine if there are any predictive factors that affect progress and its rate.
Studies5, 36 have previously reported visual field changes following PRP, but no study has looked at the progressive nature over a 2-year time period. The progressive improvement in ischemia following PRP is also a novel finding. Studies have never correlated changes in visual fields with optic nerve changes post PRP. For that reason, the current study sought to obtain comprehensive results, and conducted analyses of the optic nerve with both structural and functional tests to see if any early changes could be found in the optic nerve post PRP.
In conclusion, this study found that pascal photocoagulation did not cause significant changes to macular or RNFL thickness over the 24-month time period. The morphology of the optic nerve head appears unchanged following laser treatment as observed on the OCT and HRT diagnostic machines. Despite an improvement in peripheral perfusion, there was a significant progressive decline of peripheral visual field over the study period. Clinical grading of the optic nerve was more unreliable following PRP, despite the absence of significant morphological changes as detected by the OCT and HRT diagnostic machines. Additional research is needed to help guide clinicians in making a diagnosis of normal-tension glaucoma in diabetic patients who have received PRP.

References
Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35 (3): 556–564.
Lee R, Wong TY, Sabanayagam C . Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015; 2: 17.
Writing Committee for the Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015; 314 (20): 2137–2146.
Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology 1981; 88: 583–600.
Frank RN . Visual fields and electroretinography following extensive photocoagulation. Arch Ophthalmol 1975; 93 (8): 591–598.
Kim HY, Cho HK . Peripapillary retinal nerve fiber layer thickness change after panretinal photocoagulation in patients with diabetic retinopathy. Korean J Ophthalmol 2009; 23 (1): 23–26.
Muqit MMK, Marcellino GR, Henson DB, Fenerty CH, Stanga PE . Randomized clinical trial to evaluate the effects of pascal panretinal photocoagulation on macular nerve fiber layer. Retina 2011; 31 (8): 1699–1707.
Fujimoto JG. Optical Coherence Tomography of Ocular Diseases, 2nd ed. Thorofare Slack Inc.: Thorofare, NJ, USA, 2004, pp 714..
Mwanza J-C, Oakley JD, Budenz DL, Anderson DR . Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology 2011; 118 (2): 241–8. e1.
Foo L-L, Perera SA, Cheung CY, Allen JC, Zheng Y, Loon SC et al. Comparison of scanning laser ophthalmoscopy and high-definition optical coherence tomography measurements of optic disc parameters. Br J Ophthalmol 2012; 96 (4): 576–580.
Meditec CZ. Stratus OCT User’s Manual. Carl Zeiss Meditec: Dublin, CA, USA, 2003..
Vizzeri G, Balasubramanian M, Bowd C, Weinreb RN, Medeiros FA, Zangwill LM . Spectral domain-optical coherence tomography to detect localized retinal nerve fiber layer defects in glaucomatous eyes. Opt Express 2009; 17 (5): 4004.
Wollstein G, Garway-Heath DF, Hitchings RA . Identification of early glaucoma cases with the scanning laser ophthalmoscope. Ophthalmology 1998; 105 (8): 1557–1563.
Rao HL, Babu GJ, Sekhar GC . Comparison of the diagnostic capability of the Heidelberg retina tomographs 2 and 3 for glaucoma in the Indian population. Ophthalmology 2010; 117 (2): 275–281.
Rao H, Begum V, Addepalli U, Senthil S, Garudadri C . Optic nerve head parameters of high-definition optical coherence tomography and Heidelberg retina tomogram in perimetric and preperimetric glaucoma. Indian J Ophthalmol 2016; 64 (4): 277.
Alencar LM, Bowd C, Weinreb RN, Zangwill LM, Sample PA, Medeiros FA . Comparison of HRT-3 glaucoma probability score and subjective stereophotograph assessment for prediction of progression in glaucoma. Invest Ophthalmol Vis Sci 2008; 49 (5): 1898.
Kummet CM, Zamba KD, Doyle CK, Johnson CA, Wall M . Refinement of pointwise linear regression criteria for determining glaucoma progression. Invest Ophthalmol Vis Sci 2013; 54 (9): 6234.
Wessel MM, Aaker GD, Parlitsis G, Cho M, D'amico DJ, Kiss S . Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina 2012; 32 (4): 785–791.
Nicholson L, Vazquez-Alfageme C, Ramu J, Triantafyllopoulou I, Patrao NV, Muwas M et al. Validation of concentric rings method as a topographic measure of retinal nonperfusion in ultra-widefield fluorescein angiography. Am J Ophthalmol 2015; 160 (6): 1217–1225. e2.
Blodi BA, Domalpally A, Scott IU, Ip MS, Oden NL, Elledge J et al. Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study system for evaluation of stereoscopic color fundus photographs and fluorescein angiograms: SCORE Study Report 9. Arch Ophthalmol 2010; 128 (9): 1140–1145.
Sanghvi C, Mclauchlan R, Delgado C, Young L, Charles SJ, Marcellino G et al. Initial experience with the Pascal photocoagulator: a pilot study of 75 procedures. Br J Ophthalmol 2008; 92 (8): 1061–1064.
Muqit MMK . In vivo laser-tissue interactions and healing responses from 20- vs 100-millisecond pulse Pascal photocoagulation burns. Arch Ophthalmol 2010; 128 (4): 448.
Mahgoub MM, Macky TA . The effect of laser panretinal photocoagulation on diabetic macular edema using the Pascal photocoagulator versus the conventional argon laser photocoagulator. Ophthalmologica 2016; 235: 137–140.
Nagpal M, Marlecha S, Nagpal K . Comparison of laser photocoagulation for diabetic retinopathy using 532-nm standard laser versus multispot pattern scan laser. Retina 2010; 30 (3): 452–458.
Jain A . Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch Ophthalmol 2008; 126 (1): 78.
Shimura M, Yasuda K, Nakazawa T, Kano T, Ohta S, Tamai M . Quantifying alterations of macular thickness before and after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Ophthalmology 2003; 110 (12): 2386–2394.
Mitsch C, Pemp B, Kriechbaum K, Bolz M, Scholda C, Schmidt-Erfurth U . Retinal morphometry changes measured with spectral domain-optical coherence tomography after pan-retinal photocoagulation in patients with proliferative diabetic retinopathy. Retina 2016; 36 (6): 1162–1169.
Cui JZ, Wang X-F, Hsu L, Matsubara JA . Inflammation induced by photocoagulation laser is minimized by copper chelators. Lasers Med Sci 2008; 24 (4): 653–657.
Taguchi H, Ogura Y, Takanashi T, Hashizoe M, Honda Y . Fluorophotometric detection of intravitreal peroxides after panretinal laser photocoagulation. Invest Ophthalmol Vis Sci 1998; 39: 358–363.
Maschio AD . Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions. J Cell Biol 1996; 135 (2): 497–510.
Tsujikawa A, Kiryu J, Dong J, Yasukawa T, Suzuma I, Takagi H et al. Quantitative analysis of diabetic macular edema after scatter laser photocoagulation with the scanning retinal thickness analyzer. Retina 1999; 19 (1): 59–64.
Waisbourd M, Ahmed OM, Molineaux J, Gonzalez A, Spaeth GL, Katz LJ . Reversible structural and functional changes after intraocular pressure reduction in patients with glaucoma. Graefes Arch Clin Exp Ophthalmol 2016; 254 (6): 1159–1166.
Lim MC . Effect of diabetic retinopathy and panretinal photocoagulation on retinal nerve fiber layer and optic nerve appearance. Arch Ophthalmol 2009; 127 (7): 857–862.
Chauhan BC, Burgoyne CF . From clinical examination of the optic disc to clinical assessment of the optic nerve head: a paradigm change. Am J Ophthalmol 2013; 156 (2): 218–227. e2.
Hudson C, Flanagan JG, Turner GS, Chen HC, Young LB, Mcleod D . Influence of laser photocoagulation for clinically significant diabetic macular oedema (DMO) on short-wavelength and conventional automated perimetry. Diabetologia 1998; 41 (11): 1283–1292.
Subash M, Comyn O, Samy A, Qatarneh D, Antonakis S, Mehat M et al. The effect of multispot laser panretinal photocoagulation on retinal sensitivity and driving eligibility in patients with diabetic retinopathy. JAMA Ophthalmol 2016; 134 (6): 666–671.
Acknowledgements
We thank the Ivey Eye Institute, Western University—Department of Pathology and Laboratory Medicine, Lawson Health Research Institute, and the Schulich School of Medicine and Dentistry for their funding and support. We thank all of the secretaries, staff, and technicians at the Ivey Eye Institute for their assistance in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Meeting Presentation: ARVO 2016 Annual Meeting (Seattle, WA, USA)—Poster/Abstract presentation May 1 2016–May 5 2016. 2016 COS Annual Meeting and Exhibition (Ottawa, Ontario, Canada)—Platform presentation June 17 2016–June 20 2016.
Rights and permissions
About this article
Cite this article
Filek, R., Hooper, P., Sheidow, T. et al. Structural and functional changes to the retina and optic nerve following panretinal photocoagulation over a 2-year time period. Eye 31, 1237–1244 (2017). https://doi.org/10.1038/eye.2017.66
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.66