Abstract
Purpose
To report the incidence and associated factors for the development of vitreomacular interface abnormality (VMIA) in patients with diabetic macular edema (DME) who received intravitreal injection (IVI) of anti-VEGF (Bevacizumab and Ranibizumab) treatment.
Methods
A retrospective observational study. Patients with DME followed at least 6 months were reviewed. Baseline best-corrected visual acuity (BCVA), central retinal thickness (CRT) and final BCVA, CRT in eyes with and without VMIA were compared. Multiple logistic regression was also used to investigate the risk factors of VMIA formation in patients with DME treated by anti-VEGF.
Results
A total of 201 eyes in 142 patients met the inclusion criteria of the study. VMIA developed in 44 eyes (21.89%) of patients during a mean follow-up period of 40.84 months. The estimated mean incidence of VMIA formation was 6.43% per year. Poor baseline BCVA was found to be a risk factor for VMIA development (P=0.001, odds ratio=5.299, 95% confidence interval: 1.972 to 14.238). There was no difference between eyes with and without VMIA formation in improving BCVA (P=0.557) and lowering the macular edema (eyes without VMIA formation: −107.72±171.91 μm; eyes with VMIA formation: −155.02±212.27 μm, P=0.133).
Conclusions
This study revealed the incidence of VMIA formation in IVI anti-VEGF treated DME eyes was 6.43%. Poor baseline BCVA was found to be a risk factor for VMIA formation. Both eyes with and without VMIA development had favorable response to anti-VEGF treatment.
Similar content being viewed by others
Introduction
Diabetic macular edema (DME) is the most frequent cause of visual loss in patients with diabetic retinopathy.1 In the past, macular laser photocoagulation has been the standard treatment for DME for many years.2 Recently, intravitreal anti-VEGF injection such as bevacizumab, ranibizumab and aflibercept has been found to be an effective treatment for reducing macular edema and improving the visual acuity of patients with DME.3 Many clinical studies have revealed a beneficial effect of intravitreal injection (IVI) of anti-VEGF in both reducing macular edema and improving visual acuity in DME.4, 5
As intravitreal anti-VEGF therapy has become increasingly popular in recent clinical practice for treatment of DME, concern about possible increases in ocular complications has become an important issue. Vitreomacular interface abnormality (VMIA) such as epiretinal membrane (ERM) or vitreomacular traction (VMT) is one of the most common complications associated with ocular surgical procedures. It is well known that ocular surgeries such as cataract extraction,6, 7, 8, 9, 10, 11 surgery for retinal detachment,12, 13 laser photocoagulation7, 12 and retinal cryopexy procedures12, 14 are all associated with an increased incidence of ERM formation. As diabetic retinopathy and DME usually run a chronic and recurrent course, repeated intravitreal injection is often necessary to maintain the edema-suppressing effect. It is possible that repeated intraocular surgical procedures may have detrimental effects with formation of VMIA after a prolonged period. Although suppressing the VEGF level in PDR is beneficial for the suppression of fibrovascular membrane formation, several studies have reported paradoxical worsening of traction membranes associated with fibrovascular membranes in some patients with PDR.15, 16, 17 To the best of our knowledge, it has still not been fully elucidated whether intravitreal anti-VEGF injections for treatment of DME, either from the repeated IVI procedures or from the anti-VEGF per se, affect the incidence of VMIA formation after a prolonged period.
In this study, we retrospectively investigated the incidence of VMIA formation in a group of patients with clinically significant DME treated with intravitreal anti-VEGF for at least 6 months. The influence of VMIA formation on the treatment effect of IVI anti-VEGF for DME was also investigated. To better understand the conditions associated with the formation of VMIA in DME patients treated with IVI anti-VEGF, several systemic and ocular factors including age, gender, hypertension, hyperlipidemia, mean level of glycosylated hemoglobin (HbA1c), best-corrected visual acuity (BCVA), central retinal thickness (CRT), prior cataract surgery, macular laser treatment and pan-retinal photocoagulation (PRP) were further analyzed.
Materials and methods
Patients recruitment
We conducted a retrospective study of patients with DME who received intravitreal injection (IVI) of anti-VEGF treatment (including Bevacizumab and Ranibizumab) at Shin Kong Wu Ho-Su Memorial Hospital from January 2006 to December 2014. Institutional review board/ethics committee approval and informed consents from the patients for anti-VEGF injections were obtained. The clinical records of all consecutive patients with DME were reviewed. The diagnosis of DME was made by the presence of exudative changes and thickening in the macula on ophthalmoscopic examination and evidence of late macular leakage on the fluorescein angiography (FA). Increased CRT or cystic change was observed on optical coherence tomography (OCT, Stratus, Carl Zeiss Meditec, Dublin, CA, USA, or RTvue, Optovue Inc., Fremont, CA, USA for patients enrolled between December 2013 and 2014). (All cases were kept using the same OCT examination throughout the follow-up period for the comparison of CRT). Eyes with evidence of VMIA at the initial visit were recorded and excluded from the study. Eligible patients were enrolled into this study according to defined inclusion and exclusion criteria. Inclusion criteria for our study were: an initial diagnosis of DME without VMIA made by ophthalmoscopy, FA and OCT (with regular follow-up for at least 6 months after the first diagnosis of DME). Eyes were excluded if they had any of the following conditions: (1) VMIA or vitreous hemorrhage before IVI treatment, (2) vitrectomy prior to or within 6 months of follow up period, (3) intravitreal corticosteroid injection prior to or within 6 months of the follow-up period, (4) Vitreous hemorrhage which precluded a detailed macular examination within 6 months of the follow-up period, and (5) concomitant ocular diseases such as posterior uveitis, advanced age-related macular degeneration or retinal vascular occlusions. Macular focal or grid laser, pan-retinal photocoagulation and cataract operation at baseline and during the follow-up period were recorded as items for subsequent analysis.
Treatment protocol
All patients received at least one anti-VEGF injection according to a standard aseptic operating procedure, which has been described in a previous study.18 After injection, patients were examined 1 week after injection and then monthly thereafter. BCVA, intraocular pressure, slit-lamp biomicroscopy, dilated fundus ophthalmoscopy and OCT were performed at each visit. FA was performed only for eyes with suspected progression of diabetic retinopathy or macular ischemia. The injection was repeated at the treating physician’s discretion if the BCVA or CRT deteriorated again after the first injection.
Data collection
BCVA was recorded in Snellen units and converted to logarithm of minimal angle of resolution (Log MAR) units for statistical analysis. The values were expressed as mean±standard deviation.
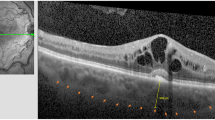
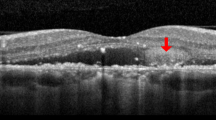
The definition of VMIA in our study was characterized as typical features of ERM or VMT on both ophthalmoscopy and OCT examinations. The ophthalmoscopic features of VMIA were detected as shining or wrinkling reflections on or above the surface of the macula. The OCT characteristics of VMIA were classified into ERM and VMT according to a recent definition by 'The International Vitreomacular Traction Study Group', which devided the vitreomacular interface features into 3 categories, namely, (1) vitreomacular adhesion (focal or broad adhesion of vitreous to inner retina without abnormal retinal changes), (2) vitreomacular traction (focal or broad adhesion of vitreous to inner retina with retinal abnormal changes), (3) Full-thickness macular hole (with/without traction; small, medium, large;19 Figure 1).20, 21 The OCT configurations of ERM were defined as partially separated or globally adherent membranes above the macular area according to the classification reported by Wilkins and associates (Figure 1).20 Partially separated ERM were detected as thin but distinctive, highly reflective bands just above the inner surface of the retina with some focal attached points. Globally adherent membranes may be detected when various combinations of the following configurations had been noted: a macular pseudohole, a difference in optical reflectivity between the membrane and retina, or a visible membrane tuft or edge. The OCT configuration of VMT were defined as having the following 3 characteristics: (1) evidence of perifoveal vitreomacular adhesion; (2) macular attachment of the vitreous cortex within a 3-mm radius of the fovea; and (3) association of attachment with distortion of the foveal surface, intraretinal structural changes, elevation of the fovea above the RPE, or a combination thereof, but no full-thickness interruption of all retinal layers. Eyes with the development of full-thickness macular hole were also recorded.
Representative optical coherence tomography (OCT) demonstrating the formation of vitreomacular interface abnormality (VMIA) in diabetic macular edema treated with intravitreal anti-VEGF (IVI). (a and b) OCT findings before (a) and after (b) development of epiretinal membrane (arrows). (c and d) OCT findings before (c) and after (d) development of VMT (arrows). (e and f) OCT findings before (e) and after (f, SD-OCT) development of ERM (arrows) with partially visible VMT (arrowhead).
Eyes were diagnosed with VMIA when an OCT configuration of ERM or VMT were detected in at least two of the six diagonal scans in the OCT examination associated with the characteristic findings previously described in the ophthalmoscopic examination, fundus photographs, or FA. Systemic conditions such as a history of hypertension, hyperlipidemia, macular grid or focal laser, PRP, and the level of HbA1c were also recorded during follow-up.
Data analysis
Wilcoxon signed-rank test was used to compare the mean age, mean HbA1c during follow-up, baseline BCVA, final BCVA, changes of BCVA, baseline CRT, final CRT, changes of CRT and the follow-up duration between patients who developed VMIA (VMIA (+) group) and who did not (VMIA (-) group). Mann–Whitney U-test was used to compare the baseline BCVA and CRT with the final BCVA and CRT in each of the two groups. We also compared the gender and the proportion of gender, hypertension, hyperlipidemia, cataract surgery, macular laser, and PRP between the two groups by the Chi-square test. Stepwise multiple logistic regression was also used to investigate the associations of the binary dependent variable 'presence of VMIA' with the continuous or categorical independent variables, such as age, hypertension, hyperlipidemia, mean HbA1c, cataract surgery, macular laser, PRP, baseline BCVA, and baseline CRT. SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA) was used.
Results
Demographic data and overall treatment outcome
A total of 201 eyes in 142 patients who fulfilled the inclusion and exclusion criteria were included in this study (86 eyes in 70 patients found to have VMIA before IVI treatment had been excluded from the study) (Table 1). The mean age was 59.90±9.29 years. There were 78 male and 64 female. The mean follow-up duration was 40.84±24.88 months. A total of 865 IVI were performed, of which bevacizumab and ranibizumab were used in 721 (83.35%) and 144 injections (16.65%) of respectively. Each eye received a mean of 4.28±3.67 injections during the follow-up period.
The mean baseline BCVA was 0.90±0.51 Log MAR units (Snellen equivalent 6/48) and the mean final BCVA was 0.73±0.50 Log MAR units (Snellen equivalent 6/32) (Table 1). The result showed that after a mean of 4.28±3.67 injections, the final BCVA was significantly better than the baseline BCVA (P=0.027), with improvement of BCVA by −0.17±0.43 Log MAR units.
The mean baseline CRT was 397.81±153.55 μm and the final CRT was 278.92±113.45 μm (Table 1). After IVI treatment, the final CRT were significantly lower than the baseline CRT (P<0.001).
Incidence and factors associated with VMIA formation
Basic characteristics in eyes that developed VMIA and that did not develop VMIA were depicted in Table 2. Forty four eyes (21.89%) developed VMIA during the follow-up period. From these, 37 (18.52%) eyes in 26 patients were predominantly of the ERM configuration (2 eyes also had partial VMT) and 7 (3.37%) eyes in 7 patients had the VMT configuration on OCT (Figure 1). We did not find any case with development of full-thickness macular hole. The estimated mean incidence22 of VMIA development (rate of VMIA formation/follow-up duration) was 6.43% per year (Table 1). The mean DME duration from the initiation of IVI to the VMIA detected was 16.27±13.75 months.
Univariate analysis revealed that only a poor baseline BCVA was significantly associated with the development of VMIA in our study (P<0.001). A thicker central subfield retinal thickness (CRT) was found to be associated with the development of VMIA with marginal significance (P=0.052; Table 2). The correlation between initial BCVA and CRT was calculated to be 0.256, which revealed a low correlation between initial BCVA and CRT. Stepwise logistic regression also revealed that a poor baseline BCVA was the only risk factor associated with VMIA formation (P=0.001, odds ratio=5.299, 95% confidence interval: 1.972 to 14.238). Other systemic or ocular factors such as age, hypertension, hyperlipidemia, HbA1c, anti-VEGF agents (percentage of bevacizumab or ranibizumab) number of injections, cataract operation, macular laser and PRP were all not significantly associated with VMIA formation (Table 3).
VMIA formation and final outcomes
The present study also revealed that VMIA formation seemed to reduce the effectiveness of anti-VEGF in the treatment of DME to a certain degree. Eyes without VMIA formation had significant improvement of BCVA from the baseline to final BCVA (0.83±0.48 Log MAR units to 0.64±0.45 Log MAR units, P<0.001), while in eyes with VMIA formation improvement of BCVA did not reach statistical significance (1.17±0.51 Log MAR units to 1.03±0.53 Log MAR units, P=0.088; Figure 2a). However, the changes from the baseline to the final BCVA were not significantly different (P=0.557) between eyes without VMIA formation (improved by −0.18±0.39 Log MAR units) and eyes with VMIA formation (improved by −0.14±0.54 Log MAR units). On the other hand, the formation of VMIA did not affect the improvement of macular edema in our study. The improvement from the baseline to final CRT was found to be statistically significant both in eyes without VMIA formation (386.52±157.78 μm to 270.74±113.34 μm, P<0.001) and in eyes with VMIA formation (439.60±130.16 μm to 311.67±109.25 μm, P<0.001) (Figure 2b). The changes from the baseline to the final CRT were not different in both groups (eyes without VMIA formation: −107.72±171.91 μm; eyes with VMIA formation: −155.02±212.27 μm, P=0.133).
(a) The baseline BCVA and final BCVA in eyes with and without VMIA formation. (b) The baseline CRT and final CRT in eyes with and without VMIA formation. *P<0.05 by Wilcoxon signed-rank test. BCVA, best-corrected visual acuity; CRT, central retinal thickness; VMIA, Vitreomacular interface abnormality; VMIA (+), eyes with VMIA formation; VMIA (−), eyes without VMIA formation.
Complications of IVI Anti-VEGF
No endophthalmitis, rhegmatogenous retinal detachment or vitreous hemorrhage was noted after any intravitreal injections. Also, no severe systemic adverse thromboembolic events such as cerebrovascular accidents, myocardial infarction or peripheral vascular disease were reported during follow-up.
Discussion
The current study investigated the incidence of the development of VMIA in DME patients with IVI Anti-VEGF treatment over a 40-month period, which, to the best of our knowledge, has rarely been reported in the literature. Our study revealed that 44 eyes (21.89%, 35 patients) of a total of 201 eyes who had been submitted to IVI treatment developed VMIA during a mean follow-up period of 40.84 months, which corresponds to an estimated mean incidence of 6.43%. Of 44 eyes that develop VMIA, 37 (18.52%) eyes were of the ERM configuration and 7 (3.37%) eyes had the features of VMT type (Figures 1c and d). The mean DME duration from the initiation of IVI to the VMIA detected was 16.27±13.75 months.
We are not aware whether the estimated incidence of 6.43% per year of the new occurrence of VMIA in our patients with IVI treated DME is higher than that of the DME patients without IVI treatment, as we did not have a well-matched control for a reliable comparison. There were also very few large-scale reports about the incidence of VMIA formation in DME patients in the literature. We previously reported an incidence of 4.42% of new VMIA formation in a group of 76 DME patients (96 eyes) without IVI treatment.18 The incidence of VMIA development of the IVI treated eyes in this study was seemingly higher than that of the non-IVI treated eyes in our previous study. However, considering that the basic characteristics were very different between patients in each group, it is still inconclusive whether or not IVI anti-VEGF would increase the incidence of VMIA formation.
Controversy exists regarding the effect of intravitreal anti-VEGF on the development or progression of fibrous membranes. As VEGF has been found to have an important role in the formation of VMIA in patients with diabetic retinopathy,23, 24 it is reasonable to postulate that IVI anti-VEGF may have an inhibitory effect on subsequent VMIA formation in most instances. However, anti-VEGF has been reported to lead to shrinkage and contracture of the fibrovascular membrane in diabetic retinopathy15, 17and in retinopathy of prematurity;25, 26 this has raised concern of the possibility that IVI anti-VEGF may paradoxically increase the incidence of VMIA formation in DME patients. Arevalo et al16 had reported that this paradoxical progression of tractional retinal detachment occurred in only 11 out of 211 patients (5.2%) with PDR after IVI in a retrospective analysis. Considering the complex pathogenesis associated with the progression of the traction membrane in PDR, this percentage probably might not be sufficient to support that intravitreal anti-VEGF is a major factor for inducing the progression of tractional epiretinal membrane in eyes with diabetic retinopathy.
DME has been found to be associated with a high prevalence of VMIA (15.6–52.1%), and the prevalence seems to increase in proportion to the severity of DME.21, 27, 28 Our studies investigated a group of eyes with clinically significant diabetic macular edema in which a substantial proportion (38.31%) did not respond well to the macular laser treatment. The studied eyes might be inherently prone to the development of VMIA. Thus, the occurrence of VMIA in 21.89% of eyes in our study after a mean of 40.84 months with IVI anti-VEGF treatment probably were not particularly high compared with the previous studies.
Our study also investigated the possible risk factors associated with the development of VMIA in DME patients with IVI anti-VEGF treatment. Older age has been identified as the most consistent risk factor associated with the formation of idiopathic ERM in many large population-based studies.6, 10, 29, 30, 31 A previous study from our group also revealed that older age was associated with development of VMIA in a group of less severe DME without IVI treatment.18 However, we observed no correlation between age and VMIA (most were of the ERM configuration) formation in DME patients with IVI treatment in this study. In the present study, initially poor BCVA was found to be the most important factor associated with the development of VMIA, either by using univariate analysis (P<0.001) or by stepwise logistic regression analysis (P=0.001, odds ratio=5.299, 95% confidence interval: 1.972–14.238). Initial CRT was also found to be the second important factor associated with the VMIA formation during anti-VEGF treatment, however, the significance of this factor is only marginal (P=0.052). There was only a low correlation between the initial BCVA and CRT in our study. It is possible that as most eyes had severe or persistent clinically significant DME with high CRT, further increase in CRT may not exert further influence on the development of VMIA. Also the detection of VMIA may be more difficult in those patients with high CRT, which may result in underestimation of the contribution of CRT to the development of VMIA.
In the literature, cataract operation and thermal laser photocoagulation such as PRP has been well known to be associated with higher prevalence of VMIA formation.6, 7, 8, 9, 10, 11, 12 However, in our study, these were not found to be associated with VMIA formation (Table 2). It is possible that our study size was too small to have enough statistical power to detect categorized risk factors for VMIA such as percentage of cataract operation or PRP for an infrequent complication like VMIA. Macular laser treatment, on the other hand, was less often found to be associated with VMIA formation.27, 28 The same result was also noted in our study (Table 2). Other systemic or ocular factors such as hypertension, hyperlipidemia, and level of HbA1c, were also found to have no significant correlation with the development of VMIA (Table 2). These factors were also less consistently found to be associated with the formation of VMIA in previous reports.6, 10, 29, 30, 31, 32, 33
The current study showed an improvement of BCVA and CRT at the final visit compared with baseline in eyes treated with IVI; the differences were statistically significant for both BCVA and CRT as expected. (BCVA: P=0.027, CRT: P<0.001). These results were not surprising as there have been many studies reporting that IVI is effective for both short-term and long-term treatment of DME. However, this study also revealed that anti-VEGF was less effective in improving the BCVA after the formation of VMIA. The improvement in BCVA was no longer significant in the group with VMIA formation (P=0.088) compared with the group without VMIA formation (P<0.001; Figure 2a), although the decrease in CRT in OCT findings were not significantly different between these two groups. Whether the existence of VMIA would influence the therapeutic effect of anti-VEGF on DME were not consistent in previous reports. Several studies reported that the effect of anti-VEGF was reduced in the treatment of DME with VMIA formation,34, 35 while Sadiq et al36 in a recent prospective study (READ-3) reported that DME patients with vitreomacular adhesion without actual traction had a greater potential for improvement in visual outcomes with anti-VEGF therapy. Nevertheless, in our study, as the VMIAs were all newly formed during the treatment period, the tractional force was probably still very week and not enough to affect the macular thickness.
In summary, our study revealed that patients with clinically significant DME who underwent repeated IVI had a rate of VMIA formation in 21.89% of eyes during a follow-up period of 40.84 months, or an estimated incidence of 6.43% per year. Treatment with anti-VEGF probably would not significantly increase the incidence of VMIA formation compared with previous studies. This study also revealed that initial poor vision is associated with VMIA formation, which is further associated with reduced treatment effect of anti-VEGF. However, several limitations need to be noted. First, the retrospective, non-randomized design essentially precluded the enrollment of a well-matched control for head-to-head comparison. Second, our study started from 2006 when only time-domain OCT was available. The use of a time-domain OCT instead of spectra-domain OCT in most of our cases would probably be less sensitive to detect some fine or subtle VMIA formation. However, as the detection of VMIA in our study was conducted not only on OCT alone, but also by a constellation of repeated examinations including opthalmoscopy, fundus photography and FA, the sensitivity of detection of VMIA may be compensated to a large extent. Third, a mean injection number of 4.2 during a mean follow-up of 40.8 months in our 'real world' clinical practice settings (where most patients did not receive a reimbursement from insurance system in the study period) were obviously less than the optimal injection numbers. This less-than-optimal treatment condition seems to be a frequent phenomenon in other real-world settings even in groups where patients were covered by medical insurance.37 Therefore, caution was advised when exploiting our results to other settings with more frequent and adequate IVI treatments for DME such as in randomized control trials. Nevertheless, the relative less responsiveness to IVI treatment in patients who developed VMIA revealed in this study may have clinical implications. Other treatment modalities such as vitrectomy with membrane peeling should be considered if VMIA formation severely impaired the visual and anatomic outcome. Further studies with prospective and case controlled designs are needed to verify these conclusions.

References
Zimmet P, Alberti KG, Shaw J . Global and societal implications of the diabetes epidemic. Nature 2001; 414 (6865): 782–787.
ETDRS Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985; 103 (12): 1796–1806.
Bandello F, Cunha-Vaz J, Chong NV, Lang GE, Massin P, Mitchell P et al. New approaches for the treatment of diabetic macular oedema: recommendations by an expert panel. Eye 2012; 26 (4): 485–493.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015; 372 (13): 1193–1203.
Elman MJ, Ayala A, Bressler NM, Browning D, Flaxel CJ, Glassman AR et al. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015; 122 (2): 375–381.
Mitchell P, Smith W, Chey T, Wang JJ, Chang A . Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology 1997; 104 (6): 1033–1040.
Mester U, Volker B, Kroll P, Berg P . Complications of prophylactic argon laser treatment of retinal breaks and degenerations in 2,000 eyes. Ophthalmic Surg 1988; 19 (7): 482–484.
Jahn CE, Minich V, Moldaschel S, Stahl B, Jedelhauser P, Kremer G et al. Epiretinal membranes after extracapsular cataract surgery(1). J Cataract Refract Surg 2001; 27 (5): 753–760.
Fraser-Bell S, Guzowski M, Rochtchina E, Wang JJ, Mitchell P . Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology 2003; 110 (1): 34–40.
Fraser-Bell S, Ying-Lai M, Klein R, Varma R . Prevalence and associations of epiretinal membranes in latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci 2004; 45 (6): 1732–1736.
Hayashi K, Hayashi H . Influence of phacoemulsification surgery on progression of idiopathic epiretinal membrane. Eye 2009; 23 (4): 774–779.
Kraushar MF, Morse PH . The relationship between retina surgery and preretinal macular fibrosis. Ophthalmic Surg 1988; 19 (12): 843–848.
Katira RC, Zamani M, Berinstein DM, Garfinkel RA . Incidence and characteristics of macular pucker formation after primary retinal detachment repair by pars plana vitrectomy alone. Retina 2008; 28 (5): 744–748.
Campochiaro PA, Gaskin HC, Vinores SA . Retinal cryopexy stimulates traction retinal detachment formation in the presence of an ocular wound. Arch Ophthalmol 1987; 105 (11): 1567–1570.
Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M . Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2008; 246 (12): 1699–1705.
Arevalo JF, Maia M, Flynn Jr HW, Saravia M, Avery RL, Wu L et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 2008; 92 (2): 213–216.
Jonas JB, Schmidbauer M, Rensch F . Progression of tractional retinal detachment following intravitreal bevacizumab. Acta Ophthalmol 2009; 87 (5): 571–572.
Chang C-K, Cheng C-K, Bai C-H, Peng C-H, Hu C-C . Development of vitreomacular interface abnormality in patients with diabetic macular edema. Taiwan J Ophthalmol 2012; 2 (3): 93–98.
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013; 120 (12): 2611–2619.
Wilkins JR, Puliafito CA, Hee MR, Duker JS, Reichel E, Coker JG et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology 1996; 103 (12): 2142–2151.
Ghazi NG, Ciralsky JB, Shah SM, Campochiaro PA, Haller JA . Optical coherence tomography findings in persistent diabetic macular edema: the vitreomacular interface. Am J Ophthalmol 2007; 144 (5): 747–754.
Jabs DA . Improving the reporting of clinical case series. Am J Ophthalmol 2005; 139 (5): 900–905.
Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW . Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol 1996; 122 (3): 393–403.
Nam DH, Oh J, Roh JH, Huh K . Different expression of vascular endothelial growth factor and pigment epithelium-derived factor between diabetic and non-diabetic epiretinal membranes. Ophthalmologica 2009; 223 (3): 188–191.
Honda S, Hirabayashi H, Tsukahara Y, Negi A . Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2008; 246 (7): 1061–1063.
Kusaka S, Shima C, Wada K, Arahori H, Shimojyo H, Sato T et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol 2008; 92 (11): 1450–1455.
Thomas D, Bunce C, Moorman C, Laidlaw AH . Frequency and associations of a taut thickened posterior hyaloid, partial vitreomacular separation, and subretinal fluid in patients with diabetic macular edema. Retina 2005; 25 (7): 883–888.
Kim BY, Smith SD, Kaiser PK . Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol 2006; 142 (3): 405–412.
McCarty DJ, Mukesh BN, Chikani V, Wang JJ, Mitchell P, Taylor HR et al. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol 2005; 140 (2): 288–294.
You Q, Xu L, Jonas JB . Prevalence and associations of epiretinal membranes in adult Chinese: the Beijing eye study. Eye 2008; 22 (7): 874–879.
Kawasaki R, Wang JJ, Sato H, Mitchell P, Kato T, Kawata S et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata study. Eye 2009; 23 (5): 1045–1051.
Kawasaki R, Wang JJ, Mitchell P, Aung T, Saw SM, Wong TY . Racial difference in the prevalence of epiretinal membrane between Caucasians and Asians. Br J Ophthalmol 2008; 92 (10): 1320–1324.
Duan XR, Liang YB, Friedman DS, Sun LP, Wei WB, Wang JJ et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci 2009; 50 (5): 2018–2023.
Wu PC, Lai CH, Chen CL, Kuo CN . Optical coherence tomographic patterns in diabetic macula edema can predict the effects of intravitreal bevacizumab injection as primary treatment. J Ocul Pharmacol Ther 2012; 28 (1): 59–64.
Yoon D, Rusu I, Barbazetto I . Reduced effect of anti-vascular endothelial growth factor agents on diabetics with vitreomacular interface abnormalities. Int Ophthalmol 2014; 34 (4): 817–823.
Sadiq MA, Soliman MK, Sarwar S, Agarwal A, Hanout M, Demirel S et al. Effect of vitreomacular adhesion on treatment outcomes in the Ranibizumab for Edema of the Macula in Diabetes (READ-3) Study. Ophthalmology 2016; 123 (2): 324–329.
VanderBeek BL, Shah N, Parikh PC, Ma L . Trends in the care of diabetic macular edema: analysis of a National Cohort. PLoS One 2016; 11 (2): e0149450.
Acknowledgements
The approval to conduct the intravitreal injection of bevacizumab was obtained from the Institutional Review Board (IRB) of Shin Kong Wu Ho-Su Memorial Hospital (IRB no. 9709-001). The study adhered to the tenets of Declaration of Helsinki.
Author contributions
Design and conduct of the study (Cheng-Kuo Cheng); Collection, management, analysis, and interpretation of the data (Chun-Kai Chang, Cheng-Kuo Cheng, Chi-Hsien Peng); preparation, review and approval of the manuscript (Chun-Kai Chang and Cheng-Kuo Cheng).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chang, CK., Cheng, CK. & Peng, CH. The incidence and risk factors for the development of vitreomacular interface abnormality in diabetic macular edema treated with intravitreal injection of anti-VEGF. Eye 31, 762–770 (2017). https://doi.org/10.1038/eye.2016.317
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.317
This article is cited by
-
The effect of vitreomacular interface in neovascular age-related macular degeneration treated with intravitreal injection of anti-VEGF
BMC Ophthalmology (2022)
-
Vitreomacular interface abnormalities in patients with diabetic macular oedema and their implications on the response to anti-VEGF therapy
Graefe's Archive for Clinical and Experimental Ophthalmology (2018)
-
Quantitative assessment of macular contraction and vitreoretinal interface alterations in diabetic macular edema treated with intravitreal anti-VEGF injections
Graefe's Archive for Clinical and Experimental Ophthalmology (2018)