Abstract
Purpose
To report the age- and gender-adjusted prevalence rates of early and late age-related maculopathy (ARM) and associated risk factors in rural and urban Indian population.
Methods
A population-based cross-sectional study was carried out in South India between 2009 and 2011. Of the 6617 subjects ≥60 years enumerated ones, 5495 (83.04%) participated in the eye examination. A detailed history including data on demographic, socioeconomic, and ocular history was obtained. Participants underwent detailed ophthalmic evaluation including 30° 3-field photograph as per Age-Related Eye Disease Study protocol. The ARM was graded according to the International ARM Epidemiological Study Group.
Results
Age- and gender-adjusted prevalence of early ARM was 20.91% (20.86–20.94) in the rural population and 16.37% (16.32–16.42) in the urban population. Similarly, the prevalence of late ARM was 2.26% (2.24–2.29) and 2.32% (2.29–2.34) in the rural and urban population, respectively. In both rural and urban populations, risk factors that were related to both early and late ARM were age, per year increase (OR, range 1.00–1.08); middle socioeconomic status (OR, range 1.05–1.83); and smokeless tobacco (OR, range 1.11–2.21). Protective factor in both was the presence of diabetes mellitus in all ARM (OR, range 0.34–0.83). Risk factors, only in the rural arm, were female gender (OR, range 1.06–1.64), past smoker (OR, 1.14), and serum low-density lipoprotein cholesterol level (OR, 1.03).
Conclusions
The study reports smokessless tobacco as a risk factor for both early and late ARM and identified a higher prevalence of early ARM in the rural population compared with urban population.
Similar content being viewed by others
Introduction
As the longevity increases, so does the aging population, which is true for both developed and developing countries.1 It has been estimated that by 2025, the geriatric population would rise to more than 1.2 billion, of which ~840 million would be living in the developing countries.2 With such a projected increase in the geriatric population, it is expected to have proportionate increase in the prevalence of age-related macular degeneration.
In India, the prevalence of age-related maculopathy (ARM) has been reported by three population-based studies: the Andhra Pradesh Eye Disease Study (APEDS); the INDEYE study; and the Central India Eye and Medical study (CIEMS).3, 4, 5 In the APEDS and CIEMS, the sample size was calculated not to estimate the prevalence of ARM, but to estimate the prevalence of all eye diseases across subjects of all age groups (in APEDS) and among those older than 30 years (in CIEMS). In the INDEYE study, although the sample size was calculated to estimate the prevalence of ARM, the population was hybrid of rural and urban; separate prevalence rates of ARM in rural versus urban populations were not reported. Therefore, the present study—Sankara Nethralaya: Rural–Urban Age-related Macular degeneration study (SN-RAM study)—was conducted, having a proportionate sample of both the rural and urban population; the objectives were to report the age- and gender-adjusted prevalence rates of early and late ARM and associated risk factors. The early ARM included the presence of drusen and/or retinal pigment epithelium (RPE) abnormalities; and late ARM, in addition, included geographic atrophy of RPE or choroidal neovascular complex.
Materials and methods
The SN-RAM study, a population-based cross-sectional study, was conducted in India between 2009 and 2011. The institutional review board approved this study and a written consent was obtained from the subjects as per the Declaration of Helsinki.
Sample size calculation
The sample size was calculated based on the INDEYE feasibility study.6 This study reported the prevalence of late ARM in the North Indian population to be 1.4%; however, the prevalence rate among subjects older than 60 years was 2.2%, and in those older than 70 years, it was 4.6%. This study was conducted in 2003.7 Considering an increase in the prevalence rate of ARM in the population with time, we assumed that the prevalence of late ARM could be ~3.5% in those older than 60 years; therefore, keeping a design effect of 2 with precision of 80% and compliance of 80%, the estimated sample size was 6617. The sample (N=6617) was divided into rural and urban proportion based on the Census of Tamil Nadu 2001; this Census showed that for those older than 60 years, the population was divided as 59% (rural) and 41% (urban).7 The proportional sample was calculated based on rural–urban divide: 3904 (60%) rural and 2713 (40%) urban.
Study areas: rural and urban
In both rural and urban areas, the sampling method was multistage random cluster sampling; a cluster was defined as having a population of 1200–2000, and if it exceeded this number, the population was divided into two or more clusters. For rural areas, the study areas were Kancheepuram and Thiruvallur districts, and for urban area, the Chennai district. The definition of a rural area included population of <10 000 as per the Reserve Bank of India guidelines; it excluded those areas that would fall in the category of urban, as per the census of India guidelines: population density of more than 400/km2 and areas wherein more than 75% of male population were engaged in non-agricultural activities.7, 8
Enumeration
A proper mapping and listing of the households was carried out in a systematic manner to avoid omissions or duplications. A geographic map showing all major structure of the study area was prepared, and all houses were numbered serially. Family members living on the same premises and sharing a common kitchen were defined as one household. A door-to-door survey of all the households on both the sides of the street was conducted in the selected division (rural and urban) till we enumerated the calculated sample size.
Inclusion and exclusion criteria
The people aged 60 years or older or those turning 60 in the present calendar year and resident at the target address for a minimum period of 6 months were included in the study. People who resided at the target households for a period of <6 months, temporary residents (people who have permanent residence elsewhere), a resident who died after the enumeration but before examination, and an eligible resident who could not be contacted after five visits by the social worker at the residence were excluded from the study. Individuals who could not be transported to the examination center because of health reason were also excluded from the study.
Clinical examination in rural and urban areas
In urban areas, the eligible subjects were given an appointment date for a comprehensive eye examination at the hospital. All the patients were brought to the base hospital. Once the comprehensive evaluation was completed, the subjects were transported back to their residence. In rural areas, the eligible subjects were given an appointment date for an eye examination at a convenient place chosen in a village where a customized mobile van, fitted with similar kinds of equipment that were used in the base hospital, was stationed (Supplementary Figure 1). The patients who required treatment were transported to the base hospital on a given appointment date.
Clinical examination protocol
Data on demographic, socioeconomic, and ocular history were obtained from all the patients at the base hospital and in the van. The socioeconomic status was assessed using a multiple index questionnaire and the scoring was characterized as: low (score, 0–14), middle (score, 15–28), and high (score, 29–42). The questionnaire included the following variables: family possession such as refrigerator, television, washing machine, and so on, own or rented house, type of house (thatched or brick), possession of vehicle (car, scooter, etc), and other financial liability or commitment. The same questionnaire and scoring were used in another population-based study.9
A detailed questionnaire was administered regarding the medical history, a general physical examination, tobacco and alcohol consumption history, and educational and occupational history. The data in the medical history included duration and treatment of diabetes mellitus or hypertension, a family history of diabetes mellitus, and coronary artery disease. The ocular history included details of the first and the last eye examination, present or past ocular complaints, and previous treatment or ocular surgery. The tobacco and alcohol consumption history included duration, type, amount, and age at start, and present and past status. Tobacco types were classified either as a smoked tobacco or smokeless tobacco. Smoked tobacco included cigarettes, cigars, cigarillos, pipes, waterpipes, kreteks (clove cigarettes), bidis (tobacco in a tendu or temburni leaf tied with a cotton thread, and papirosy (cardboard tube tipped cigarettes), whereas smokeless tobacco included chewed-tobacco (loose leaves) and snuff (finely chopped tobacco). The blood pressure was recorded, in the sitting position, in the right arm to the nearest 2 mm Hg using the mercury sphygmomanometer (Diamond Deluxe BP apparatus, Pune, India). Two readings were taken, 5 min apart, and their mean was taken as the blood pressure.9
All the subjects underwent a detailed ophthalmic evaluation that included assessment of visual acuity and refraction using modified ETDRS chart (Light House Low Vision Products, New York, NY, USA), anterior segment examination using a slit-lamp Zeiss SL 130 (Carl Zeiss, Jena, Germany), measurement of intraocular pressure using Goldmann applanation tonometer (Zeiss AT 030 Applanation Tonometer; Carl Zeiss), and fundus examination using binocular indirect ophthalmoscope (Keeler Instruments Inc., Broomall, PA, USA). Grading of len opacities was performed using the Lens Opacities Classification System (LOCS chart III; Leo T Chylack, Harvard Medical School, Boston, MA, USA) by experienced ophthalmologists (SSP and SG). Cataract grading was carried out with dilated pupil on slit lamp while comparing it with LOCS III standard photographs.
Retinal photographs were obtained after pupillary dilatation (Carl Zeiss fundus camera; FF-450, Zeiss, Jena, Germany). The ARM was graded according to the International ARM Epidemiological Study Group and stratified into stages based on the grading in the worst eye (Supplementary Figure 2).10 The grading agreement, which was carried out by two independent observers (Retina specialists) in a masked manner, was 0.62 for early ARM and 0.87 for late ARM.
All subjects underwent estimation of fasting blood glucose by enzymatic assay (Merck Micro Lab 120 semiautomated analyzer, Darmstadt, Germany), total serum cholesterol (CHOD-POD method), high-density lipoproteins (CHOD-POD method after protein precipitation), serum triglycerides (CHOD-POD), hemoglobin (calorimetric hemoglobinometer), and packed cell volume (capillary method).
There were two groups: subjects with known diabetes and newly diagnosed subjects, during enrollment. The definition of subjects with known diabetes was to consider if they were using antidiabetic drugs, either oral or insulin or both, along with dietary recommendations. For newly diagnosed subjects, oral glucose tolerance test (OGTT) was used. First step was screening at the household: capillary blood glucose by finger prick using Accutrend Alpha (Roche Diagnostics, Rotkreuz, Switzerland) was carried out with a minimum of 8 h of overnight fasting. Second step was confirmatory in the mobile van in rural and base hospital in the urban arm, all underwent OGTT, a reading of ≥200 mg% was the cutoff point. To enhance the compliance and minimize the dropouts, fasting blood glucose was estimated even on Sundays or a day of their choice.
Definitions
Early ARM was defined as the presence of drusen (discrete whitish-yellow spots located external to the neuroretina or RPE) or the presence of drusen with RPE pigmentary abnormalities (areas of hyperpigmentation or hypopigmentation).10
Late ARM was defined as the presence of dry AMD (geographic atrophy of the RPE in the absence of neovascular AMD) or neovascular AMD (RPE detachments, which may be associated with neurosensory retinal detachment, subretinal or sub-RPE neovascular membranes, epiretinal, intraretinal, subretinal, or subpigment epithelial scar/glial tissue or fibrin-like deposits and subretinal hemorrhages not related to other retinal vascular disease).10 Supplementary Figures 1a–e shows the photographs of early to late ARM.
Statistical analysis
The crude age- and gender-specific prevalence rates of early and late ARM were assessed. Direct age standardization of our study population to the Censes of India population was conducted. The association of the variables with ARM was assessed using Student’s t-test for the continuous variables and the Pearson's χ2 test for the categorical variables. Logistic regression analysis was performed to determine the risk factors using odds ratio estimates with 95% confidence intervals (CIs). Furthermore, a stepwise multivariate regression analysis was performed with P<0.05 being required for entering the model and remaining there. SPSS software (version 13.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
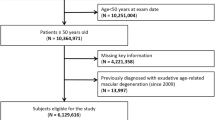
In this study, 6617 subjects were enumerated; Figure 1 flowchart shows their participation in each arm: rural, 3904; urban, 2713. Of these, 6617 enumerated persons, 5495 (83.04%) participated in the eye examination; dropout rates were 16.34% and 17.84% in the rural and urban population, respectively. Of these 5495 subjects who participated in the eye examination, 5379 (81.29%) underwent retinal imaging; 74 (rural, 60; urban, 14) were not willing for the dilatation; and 42 (rural, 32; urban, 10) were not cooperative. The retinal images were noted to be ungradable in 431 subjects in the rural population and 157 subjects in the urban population. Therefore, in the final analysis 4791 subjects (rural, 2743; urban, 2048) were included.
Table 1 shows the prevalence of early and late ARM in the rural and urban populations. Age- and gender-adjusted prevalence of early ARM was 20.91%, 95% CI (20.86–20.94) in the rural population and 16.37%, 95% CI (16.32–16.42) in the urban population. Similarly, age- and gender-adjusted prevalence of late ARM was 2.26%, 95% CI (2.24–2.29) and 2.32%, 95% CI (2.29–2.34) in the rural and urban population, respectively. No significant difference was observed gender-wise with respect to early and late ARM in both rural and urban populations.
Table 2 shows the age- and clinical parameter-wise prevalence of early ARM. In the rural population, prevalence of only drusen was 11.41%, 95% CI (11.37–11.44); drusen with hyperpigmentation, 4.89%, 95% CI (4.86–4.91); and drusen with hypopigmentation, 4.61%, 95% CI (4.59–4.63). In the urban population, prevalence of only drusen was 9.42%, 95% CI (9.38–9.46); drusen with hyperpigmentation, 4.77%, 95% CI (4.74–4.79); and drusen with hypopigmentation was 2.18%, 95% CI (2.16–2.20). In the urban population, the crude prevalence of early ARM showed increasing trend with increasing age (trend P-value=0.012); no such trend was observed in the rural population.
We did a comparison of baseline characteristics between those without ARM vs early or late ARM, in both rural and urban population (Supplementary Table 1). In the rural arm, compared with those without ARM, subjects with late ARM were older (mean age, 65.13 vs 68.49 years); less prevalence of diabetes mellitus was observed in both early ARM (24.7% vs 14%) and late ARM (24.7% vs 9.4%). Similarly, in the urban arm, compared with those without ARM, subjects with early and late ARM were older (early: 66.35 vs 67.52 years; late: 66.35 vs 70.38 years).
With regard to consumption of tobacco, there were two types of users: smokers and smokeless tobacco users. In the rural arm, smokers were 13.99% (384/2743) and smokeless tobacco users were 27.96% (767/2743); similarly, in the urban arm, smokers were 10.25% (210/2048) and smokeless tobacco users were 13.72% (281/2048).
Table 3 identifies the risk-protective factors influencing early or late ARM, as evaluated by multivariate analysis. All the parameters that were analyzed in the univariate model were included in the final model. In both rural and urban populations, three factors were related to both early and late ARM: age, per-year increase (OR, range 1.00–1.08); middle socioeconomic status (OR, range 1.05–1.83); and use of smokeless tobacco (OR, range 1.11–2.21). Use of alcohol was related to all forms of ARM in both the arms, except for late ARM in rural (OR, range 1.35–1.75). Other risk factors included female gender, only in the rural arm (OR, range 1.06–1.64); serum low-density lipoprotein (LDL) cholesterol level, only for late ARM in the rural arm (OR, 1.03); and higher socioeconomic status, only for early ARM in the urban arm (OR, 1.60). The protective factors were the presence of diabetes mellitus in all ARM, in both the arms (OR, range 0.34–0.83), and higher socioeconomic status, only in early ARM of the rural arm (OR, 0.63).
Discussion
The present study found that the prevalence of early ARM was ~21% in the rural population and ~16% in the urban population. Similarly, the prevalence of late ARM was ~2% in both rural and urban populations. The predominant lesion in early ARM, in both rural and urban populations, was only drusens with no pigment abnormalities. The prevalence of drusens with pigmentary abnormalities such as hyper- or hypopigmentation was noted in ~5% of the population in the rural subjects, and in urban subjects, the prevalence of hyperpigmentation was more compared with that of hypopigmentation (5% vs 2%). Previous epidemiological studies have shown that the presence of drusens and associated pigment abnormalities were associated with progression to late ARM.11, 12 Therefore, it is imperative to follow and educate all these patients who have shown evidence of early ARM, that is, the population at risk, 2 out of 10 subjects above the age of 60 years.
We compared the results of this study with those published in India (Supplementary Table 2). The prevalence of early ARM in the present study was higher, ranging from ~16% in urban to ~21% in rural, when compared with 8% with early ARM and 1% of late ARM in the central India study; the INDEYE study showed a high prevalence of 47% of early ARM and 1% of late ARM.3, 4 However, in the CIEMS, the sample size was not powered to estimate the correct prevalence of ARM, and in the INDEYE study, ~40% or more of the images were ungradable, a possible reason for such high prevalence of early ARM in their study. Other reasons for differences could be because of regional ethnic differences and use of definitions of ARM.13
Table 4 compares the prevalence of early and late ARM in the present study with other Asian, Western, and European population.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 The prevalence rates of late ARM in the SN-RAM study were comparable with those of European (2.29–2.5%), Asian (0.1–1.9%), and North American studies (1.1–2.1%). Similarly, the prevalence of early ARM were comparable with those of the European (15.4–29.5%) and North American studies (14.1–20%). The differences in prevalence in different populations could be explained partly by genetic differences. For example, the frequency of the C-risk allele of the Y402H polymorphism is disproportionately much lower in the Japanese when compared with the Caucasians (7% vs 34%).25, 26
We found higher prevalence of early ARM in rural population. Similar to our study, Piermarocchi et al27 also found that the rural group showed a higher risk of developing large drusen when compared with the urban sample in Italy (OR = 1.61; 95% CI: 1.01–2.63). However, in the rural Italian population, Pagliarini et al28 reported lower prevalence of ARM in the self-sustained farming community. Klein et al29 have attributed a higher rate of nitrate–nitrogen exposure from the rural private drinking water to increase the odds of both early ARM OR 2.88 (95% CI: 1.59–5.23) and late ARM OR 2.80 (95% CI: 1.07–7.31) in the rural population. The association between increasing age and ARM was noted in this study and so were the results reported by others.11, 12, 30, 31
While smoking—as a risk factor—has been associated with ARM in other populations,30, 31 we found association of smokeless tobacco use as risk factor in both urban and rural populations. Of the estimated 852 million tobacco users, globally, nearly 301 million were in China and 275 million in India.32 We observed that the prevalence of smokeless tobacco, in this study, was almost two times in the rural population when compared with urban population (27.9% vs 13.7%), and this could be the probable reason for higher prevalence of early ARM in the rural compared with that in the urban population (~21% vs 16%). Smokeless tobacco either through the direct toxic effect or through other contaminants may cause the changes leading to the increased risk of ARM. It is speculated that smokeless tobacco brings about molecular and pathological changes—vascular inflammation, endothelial dysregulation, oxidative, and toxic damage—favoring the development of ARM.33 Verghese et al34 have reported ERG changes after using nicotine gums, thus providing some evidence on the probable toxic effect of nicotine on the retina.
We found middle socioeconomic status as a risk factor for ARM in both arms. There have been conflicting reports on the relationship between socioeconomic status and ARM: the National Health and Nutrition Examination Survey (NHANES) and Eye Disease Case–Control Study (EDCCS) found an inverse relation between socioeconomic status and ARM,35, 36 whereas Beaver Dam Eye Study (BDES) found no association.23 Although the exact reasons for these differences are not known, it is likely that the differences in methods of determination of socioeconomic status might contribute to it. NHANES used education and income as the criterion of socioeconomic status, whereas BDES used education, income, and employment status as a measure of socioeconomic status.23, 35, 36
Similar to the Los Angles Latino Eye Study and Melbourne Collaborative Cohort Study (MCCS),37, 38 we found a positive association of ARM and alcohol consumption. However, this association was not found for late ARM in the rural arm. Kumar et al39 has reported a lower prevalence (9.4%) of alcohol use in rural Tamil Nadu, and also found that two-thirds of alcohol users belonged to the age group of 15–44 years. This may explain the lack of association of alcohol and ARM in late ARM in the rural arm.
There are conflicting reports regarding gender and risk of ARM.40, 41 We found no association in all arms except that women had higher odds for late ARM in the rural arm. This rural–urban difference can possibly be because of more exposure of biomass fuel among rural women. Dutta et al41 has reported that biomass users had more particulate pollution in indoor air, their serum contained significantly elevated levels of IL-6, IL-8, TNF-α, ROS, and CRP, and thus increasing oxidative stress contributing to the development of ARM.42
Similar to Reynolds et al,43 we also found elevated LDL to be associated with the increased risk of late ARM. The oxidized low-density lipoprotein could promote senescence of retinal pigment epithelial cells, which can cause outer blood–retinal barrier (BRB) dysfunction leading to changes of ARM.44 However, we found this association only in the rural arm.
Although diabetes-related changes in the function and structure of the RPE, Bruch membrane, and the choroidal circulation have been hypothesized to increase the risk of ARM,45 as seen in studies such as the European Eye study (Eureye study),46 the present study found a protective effect of the presence of diabetes on both early and late ARM. Similar to our study, a retrospective study by Borrone et al46 found that the prevalence of ARM was lower in diabetic patients and even much lower in patients with retinopathy, but the exudative form (CNV) was higher compared with the atrophic form in diabetic patients when compared with the general population.47 The early stages of diabetic retinopathy begin with alterations of the inner BRB, whereas ARM involves the outer BRB. Sander et al47 has suggested that in diabetic macular edema, there is signaling from the damaged inner BRB that induces upregulation of the transport function of the RPE (outer BRB), thus delaying the development of the ARM.48
The strengths of this study included the ability to report concurrently the prevalence rates of ARM both in the rural and urban populations of India using standardized protocol and the photographic documentation of the macula. The sample population, both in urban and rural studies, were of similar South Indian ethnicity, thus ensuring homogenous sample. However, being a cross-sectional study, risk-protective factors identified in the study represented just an association; a cause-effect relationship can only be established by a longitudinal study. Although the sample was representative of Indian population, it was not calculated separately for rural and urban populations.
On extrapolation of the findings of the present study to the Indian population based on 2011 census,7 there are ~2.38 million cases of late ARM (1.68 million rural and 0.7 million urban population). Furthermore, the subjects with early ARM who were at risk for conversion to late ARM were ~20.35 million cases (15.34 million rural and 5.01 million urban). Therefore, there is a need for public education regarding the importance of regular examination to detect early ARM changes in both rural and urban populations. The study identified two modifiable risk factors for ARM: systolic blood pressure and use of smokeless tobacco. Furthermore, early ARM was more in those with higher socioeconomic group in urban population; hence, there could be a role in modifying lifestyle in reducing the burden of blindness from ARM in the elderly population. In summary, the results of the present study do highlight that aging population is at a risk of developing ARM and every efforts must be made for its prevention and early diagnosis so as to minimize visual morbidity. These measures include a good control of systolic blood pressure, stopping tobacco intake, and conducting health education seminars for public, highlighting the importance of early screening including the use of home Amsler charting.

References
WHO, Tufts University School of Nutrition and Policy Keep Fit for Life: Meeting the Nutritional Needs of Older Persons. WHO: Geneva, Switzerland, 2002. Available at: http://whqlibdoc.who.int/publications/9241562102.pdf (last accessed on 24 September 2012).
Irudaya RS, Sarma PS, Mishra US . Demography of Indian Aging, 2001–2051. In: liebig PS, Irudaya RS (eds), An Aging India: Perspectives, Prospects, and Policies. Rawat Publications (Indian reprint): Jaipur, Rajasthan, India, 2005, pp 11–26.
Nangia V, Jonas JB, Kulkarni M, Matin A . Prevalence of age-related macular degeneration in rural central India: the Central India Eye and Medical Study. Retina 2011; 31: 1179–1185.
Krishnan T, Ravindran RD, Murthy GV, Vashist P, Fitzpatrick KE, Thulasiraj RD et al. Prevalence of early and late age-related macular degeneration in India: the INDEYE study. Invest Ophthalmol Vis Sci 2010; 51: 701–707.
Krishnaiah S, Das T, Nirmalan PK, Nutheti R, Shamanna BR, Rao GN et al. Risk factors for age-related macular degeneration: findings from the Andhra Pradesh eye disease study in South India. Invest Ophthalmol Vis Sci 2005; 46: 4442–4449.
Gupta SK, Murthy GVS, Morrison N, Dherani M, John N . Prevalence of early and late age-related macular degeneration in a rural population in Northern India: The INDEYE feasibility study. Invest Ophthalmol Vis Sci 2007; 48: 1007–1011.
Census of India 2001: Population projections for India and states 2001–2026. Available at: http://gujhealth.gov.in/basicstatastics/pdf/Projection_Report.pdf. last accessed on june 2009.
Handbook of Statistics on Indian Economy, 2011–2012; page 395, published by Reserve Bank of India. Available at: http://rbidocs.rbi.org.in/rdocs/Publications/PDFs/00HB130912LF.pdf. last accessed on October 2012.
Agarwal S, Raman R, Paul PG, Rani PK, Uthra S, Gayathree R et al. Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS 1): study design and research methodology. Ophthalmic Epidemiol 2005; 12: 143–153.
The International ARM Epidemiological Study Group. An international classification and grading system for age related maculopathy and age related macular degeneration. Surv Ophthalmol 1995; 39: 367–374.
Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH . Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 2002; 109: 1767–1779.
Wang JJ, Foran S, Smith W, Mitchell P . Risk of age-related macular degeneration in eyes with macular drusen or hyperpigmentation: the Blue Mountains Eye Study cohort. Arch Ophthalmol 2003; 121: 658–663.
Klein R, Meuer S, Myers C, Buitendijk GH, Rochtchina E, Choudhury F et al. Harmonizing the classification of age-related macular degeneration in the three continent ARM consortium. Ophthalmic Epidemiol 2014; 21 (1): 14–23.
Li Y, Xu L, Jonas JB, Yang H, Ma Y, Li J . Prevalence of age-related maculopathy in the adult population in China: the Beijing eye study. Am J Ophthalmol 2006; 142: 788–793.
Kawasaki R, Wang JJ, Ji GJ, Taylor B, Oizumi T, Daimon M et al. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population: the Funagata study. Ophthalmology 2008; 115: 1376–1381.
Oshima Y, Ishibashi T, Murata T, Tahara Y, Kiyohara Y, Kubota T et al. Prevalence of age related maculopathy in a representative Japanese population: the Hisayama study. Br J Ophthalmol 2001; 85: 1153–1157.
Chen SJ, Cheng CY, Peng KL, Li AF, Hsu WM, Liu JH et al. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci 2008; 49: 3126–3133.
Kawasaki R, Wang JJ, Aung T, Tan DT, Mitchell P, Sandar M et al. Prevalence of age-related macular degeneration in a Malay population: the Singapore Malay Eye Study. Ophthalmology 2008; 115: 1735–1741.
Yang K, Liang YB, Gao LQ, Peng Y, Shen R, Duan XR et al. Prevalence of age-related macular degeneration in a rural Chinese population: the Handan Eye Study. Ophthalmology 2011; 118: 1395–1401.
Mitchell P, Smith W, Attebo K, Wang JJ . Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology 1995; 102 (10): 1450–1460.
Augood CA, Vingerling JR, de Jong PT, Chakravarthy U, Seland J, Soubrane G et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE). Arch Ophthalmol 2006; 124: 529–535.
Erke MG, Bertelsen G, Peto T, Sjølie AK, Lindekleiv H, Njølstad I . Prevalence of age-related macular degeneration in elderly Caucasians: the Tromso Eye Study. Ophthalmology 2012; 119: 1737–1743.
Klein R, Klein BE, Linton KL . Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992; 99: 933–943.
Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM . Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology 1999; 106: 1049–1055.
Grassi MA, Fingert JH, Scheetz TE, Roos BR, Ritch R, West SK et al. Ethnic variation in AMD-associated complement factor H polymorphism p.Tyr402His. Hum Mutat 2006; 27 (9): 921–925.
Oshima Y, Ishibashi T, Murata T, Tahara Y, Kiyohara Y, Kubota T . Prevalence of age related maculopathy in a representative Japanese population: the Hisayama study. Br J Ophthalmol 2001; 85 (10): 1153–1157.
Piermarocchi S, Segato T, Scopa P, Masetto M, Ceca S, Cavarzeran F et al. The prevalence of age-related macular degeneration in Italy (PAMDI) study: report 1. Ophthalmic Epidemiol 2011; 18: 129–136.
Pagliarini S, Moramarco A, Wormald RP, Piguet B, Carresi C, Balacco-Gabrieli C et al. Age-related macular disease in rural southern Italy. Arch Ophthalmol 1997; 115: 616–622.
Klein BE, McElroy JA, Klein R, Howard KP, Lee KE . Nitrate–nitrogen levels in rural drinking water: Is there an association with age-related macular degeneration? J Environ Sci Health A Tox Hazard Subst Environ Eng 2013; 48: 1757–1763.
Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol 1980; 24: 335–610.
Schachat AP, Hyman L, Leske MC, Connell AM, Wu SY . Features of age-related macular degeneration in a black population. The Barbados Eye Study Group. Arch Ophthalmol 1995; 113: 728–735.
Thakur JS, Prinja S, Bhatnagar N, Rana S, Sinha DN . Socioeconomic inequality in the prevalence of smoking and smokeless tobacco use in India. Asian Pac J Cancer Prev 2013; 14: 6965–6969.
Velilla S, García-Medina JJ, García-Layana A, Dolz-Marco R, Pons-Vázquez S, Pinazo-Durán MD et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol 2013; 2013: 895147.
Varghese SB, Reid JC, Hartmann EE, Keyser KT . The effects of nicotine on the human electroretinogram. Invest Ophthalmol Vis Sci 2011; 52 (13): 9445–9451.
Zhang X, Cotch MF, Ryskulova A, Primo SA, Nair P, Chou CF et al. Vision health disparities in the United States by race/ethnicity, education, and economic status: findings from two nationally representative surveys. Am J Ophthalmol 2012; 154: S53–S62.
Fraser-Bell S, Wu J, Klein R, Azen SP, Varma R, Eye Study Group. Smoking, alcohol intake, estrogen use and age-related macular degeneration in Latinos: The Los Angles Latino Eye Study. Am J Ophthalmol 2006; 141: 79–87.
Adams MK, Chong EW, Williamson E, Aung KZ, Makeyeva GA, Giles GG et al. 20/20—Alcohol and age-related macular degeneration: the Melbourne Collaborative Cohort Study. Am J Epidemiol 2012; 176 (4): 289–298.
Kumar SG, Premarajan KC, Sunitha L, Suguna E, Vinayagamoorthy, Kumar V . Prevalence and pattern of alcohol consumption using Alcohol Use Disorders Identification Test (AUDIT) in Rural Tamil Nadu, India. J Clin Diagn Res 2013; 7 (8): 1637–1639.
Evans JR . Risk factors for age-related macular degeneration. Prog Retin Eye Res 2001; 20 (2): 227–253.
Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol 2010; 10: 31.
Dutta A, Ray MR, Banerjee A . Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol Appl Pharmacol 2012; 261 (3): 255–262.
Reynolds R, Rosner B, Seddon JM . Serum lipid biomarkers and hepatic lipase gene associations with age-related macular degeneration. Ophthalmology 2010; 117 (10): 1989–1995.
Kim JH, Lee SJ, Kim KW, Yu YS, Kim JH . Oxidized low density lipoprotein-induced senescence of retinal pigment epithelial cells is followed by outer blood–retinal barrier dysfunction. Int J Biochem Cell Biol 2012; 44 (5): 808–814.
Choi JK, Lym YL, Moon JW, Shin HJ, Cho B . Diabetes mellitus and early age-related macular degeneration. Arch Ophthalmol 2011; 129: 196–199.
Topouzis F, Anastasopoulos E, Augood C, Bentham GC, Chakravarthy U, de Jong PT et al. Association of diabetes with age-related macular degeneration in the EUREYE study. Br J Ophthalmol 2009; 93: 1037–1041.
Borrone R, Saravia M, Bar D . Age related maculopathy and diabetes. Eur J Ophthalmol 2008; 18: 949–954.
Sander B, Larsen M, Moldow B, Lund-Andersen H . Diabetic macular edema: passive and active transport of fluorescein through the blood–retina barrier. Invest Ophthalmol Vis Sci 2001; 42: 433–438.
Nirmalan PK, Katz J, Robin AL, Tielsch JM, Namperumalsamy P, Kim R et al. Prevalence of vitreoretinal disorders in a rural population of southern India: the Aravind Comprehensive Eye Study. Arch Ophthalmol 2004; 122: 581–586.
Acknowledgements
This work was funded by Jamshetji Tata trust, Mumbai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Eye website
Rights and permissions
About this article
Cite this article
Raman, R., Pal, S., Ganesan, S. et al. The prevalence and risk factors for age-related macular degeneration in rural–urban India, Sankara Nethralaya Rural–Urban Age-related Macular degeneration study, Report No. 1. Eye 30, 688–697 (2016). https://doi.org/10.1038/eye.2016.14
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.14
This article is cited by
-
Evaluation of the effect of smokeless tobacco (Maras powder) on choroidal and retinal blood flow: an optical coherence tomography angiography study
International Ophthalmology (2020)
-
Prevalence and causes of low vision and blindness in an elderly population in Nepal: the Bhaktapur retina study
BMC Ophthalmology (2018)
-
Age-related macular degeneration in a South Indian population, with and without diabetes
Eye (2017)
-
Prevalence and the risk factors for visual impairment in age-related macular degeneration
Eye (2017)