Abstract
Objective
The objective of this study was to evaluate the clinical effects of modified partial pars plana vitrectomy together with phacoemulsification, intraocular lens (IOL) implantation, posterior capsulectomy, and zonulohyaloidectomy for patients with malignant glaucoma after trabeculectomy or cataract surgery.
Design
Retrospective, cohort study.
Participants
Thirty consecutive patients (30 eyes) with malignant glaucoma after trabeculectomy surgery or ultrasonic phacoemulsification of cataract between January 2008 and September 2014 were enrolled.
Methods
A retrospective analysis of 30 eyes with malignant glaucoma after trabeculectomy surgery for angle-closure glaucoma or ultrasonic phacoemulsification of cataract was performed. All patients underwent modified partial pars plana vitrectomy with zonulohyaloidectomy. Phacoemulsification and IOL implantation was performed in 25 patients with no previous cataract surgery. Pre-operative and post-operative ocular parameters were recorded in detail.
Main outcome measures
Clinical features, anterior chamber depth, best-corrected visual acuity, and intraocular pressure (IOP).
Results
In these 30 patients, 25 had undergone trabeculectomy surgery and 5 had undergone cataract surgery. The mean axial length was 21.3±0.8 mm. After surgery, mean IOP decreased from 34±8.3 mm Hg to 10.5±4.1 mm Hg (P<0.001), and mean anterior chamber depth increased from 0.8±0.4 mm to 2.7±0.3 mm (P<0.001). No severe complications occurred.
Conclusions
Modified partial pars plana vitrectomy combined with phacoemulsification, IOL implantation, posterior capsulectomy, and zonulohyaloidectomy not only simplifies the process of traditional vitrectomy, but effectively resolves the ciliary block and corrects the misdirection of aqueous humor in malignant glaucoma.
Similar content being viewed by others
Introduction
Malignant glaucoma is a rare but serious complication which occurs after intraocular surgery. It is characterized by central and peripheral shallowing to flattening of the anterior chamber (AC) and significantly elevated intraocular pressure (IOP) without suprachoroidal effusion or hemorrhage in the presence of a patent iridotomy. It occurs most frequently after simple or combined filtration surgery for angle-closure glaucoma, with an incidence rate of up to 4%, but can also occur after other ocular interventions such as phacoemulsification, laser iridotomy, laser capsulotomy or cyclophotocoagulation. This complication can develop within a few days, weeks, months or even a year or more after surgery. The etiology of malignant glaucoma is believed to be related to anatomical obstruction of aqueous flow at the anterior hyaloid/zonule–lens/ciliary process interface1 which leads to the misdirection of aqueous humor into the vitreous cavity.2 Therefore, it is also referred to as ‘aqueous misdirection syndrome’, ‘ciliary block glaucoma’, and ‘ciliovitreal block glaucoma’ based on its presumed pathophysiological mechanisms.3
Therapeutic strategies for malignant glaucoma include drug therapy, laser treatment, and surgical interventions. Medications including cycloplegics/mydriatics, anti-glaucoma agents, and intravenous hyperosmotic agents are commonly used as first-line treatment. If there is no response to these therapies, laser treatment such as laser capsulotomy, laser anterior hyaloidectomy, argon cyclophotocoagulation,4 and transscleral cyclodiode laser photocoagulation5 have been used. In cases resistant to medical or laser treatment, surgery is the final treatment choice.6 Surgical treatments involve vitreous aspiration,7 core vitrectomy,8, 9 and anterior vitrectomy.10 The recurrence rate varies.8, 11, 12 During the last several years, we have modified the classic pars plana vitrectomy (PPV) which we have performed in a series of patients who were diagnosed with malignant glaucoma onset after trabeculectomy surgery. The surgery includes partial PPV, phacoemulsification, intraocular lens (IOL) implantation, posterior capsulectomy, and zonulohyaloidectomy. During the entire procedure, only one 23-gauge vitreous cutter is used without any infusion. We hope that our experience will help to simplify the surgical procedure to obtain a satisfactory curative effect.
Subjects and methods
Thirty consecutive patients (thirty eyes) who were diagnosed with malignant glaucoma after trabeculectomy surgery or ultrasonic phacoemulsification of cataract from January 2008 to September 2014 in the Department of Ophthalmology, the Ninth People's Hospital affiliated with Shanghai JiaoTong University, Shanghai, China were included in this study. Twenty-five patients had undergone trabeculectomy surgery and five had undergone cataract surgery. Of the patients who had undergone trabeculectomy, the indication for this procedure was acute angle-closure glaucoma in 7 and chronic angle-closure glaucoma in 18. The patients who had undergone cataract surgery were all judged to be in the pre-clinical stage of acute angle-closure glaucoma and had undergone YAG laser iridectomy. The diagnosis of malignant glaucoma after previous surgery was established based on the presence of a shallow or flat AC, high IOP, the presence of a patent peripheral iridotomy, and absence of choroidal effusion or suprachoroidal hemorrhage. Conventional mydriatic and IOP-lowering medications resulted in a poor response in these patients. Detailed ophthalmological examinations were carried out the day before our intervention, and included best-corrected visual acuity (VA), Goldmann applanation tonometry, slip-lamp examination, and fundus examination. VA was measured using a decimal chart and was converted to LogMAR for computing purposes. Lens opacification was evaluated. Axial length of the globe was measured by A-scan ultrasonography and the AC depth was measured by an ultrasound biomicroscope (UBM, SW3200, Suoer, China) in each patient. For patients who were expected to undergo cataract surgery at the same time, the power of the post chamber IOL was also measured in preparation for IOL implantation. Previous ocular history and topical or general anti-glaucoma medication were recorded. Patient's demagraphic data were recorded in Table 1. All 30 patients then underwent partial PPV together with posterior capsulectomy and zonulohyaloidectomy. In addition to the surgical steps previously mentioned, phakic eyes received phacoemulsification and IOL implantation. Fundus examination and B-scan ultrasonography were carried out to exclude retinal or choroidal detachment the day after surgery. The post-operative follow-up period was 6 months for all patients. Ocular parameters such as IOP, VA, complications, and medications were recorded at each visit. AC depth was also measured by UBM at each visit from the first week after surgery.
Surgical procedure
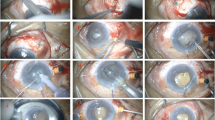
All surgeries were performed by one surgeon. The entire procedure consisted of the following three steps:
(1) Partial PPV
Following routine disinfection and retrobulbar anesthesia, a 23-gauge vitrector was inserted into the vitreous cavity via a self-healing incision ~3.5 mm from the limbus on the same median as the pre-existing peripheral iridectomy. No infusion or illumination was required. Partial vitrectomy was then performed until ~0.5 ml vitreous was removed from the anterior vitreous cavity just behind the position of the iridectomy. Normally at this time, there is an impression that the globe has softened. The purpose of this procedure was to deepen the AC by reducing the forward pressure and to soften the globe.
(2) Phacoemulsification, IOL implantation, and repetition of partial PPV
This step was performed for phakic patients only. A self-sealing transparent corneal incision was made and the AC was deepened by the injection of a viscoelastic agent. In this step, the adhesion between the iris and cornea was separated. Routine capsulorhexis was then performed. The exudative lemma in the pupil, if present, was also removed using microscissors. Then hydrodissection and phacoemulsification were performed and a foldable IOL (Allergan, Inc., Irvine, CA, USA) was inserted into the capsular bag. A partial vitrectomy was performed again via the pars plana incision to confirm that the vitreous body behind the peripheral iridectomy was cut.
(3) Zonulohyaloidectomy and posterior capsulectomy
The vitrector was inserted into the vitreous cavity via the pars plana behind the peripheral iridectomy incision. The tip of the cutter was carefully introduced through the iridectomy incision in front of the AC to achieve a zonulohyaloidectomy. Finally, in the central zone, the posterior capsule ~4 mm in diameter was cut using the vitrector to prevent the occurrence of posterior capsule opacity. In all eyes a significant deepening of the AC due to the flow of aqueous fluid was observed. The AC was filled with viscoelastic material (Viscoat, Alcon Laboratories, Inc., TX, USA). Dexamethasone 2 mg was given by subconjunctival injection, and both topical levofloxacin (Kelobituo; Santen Pharmaceutical Co., Osaka, Japan) and tropicamide (Meiduoli; Santen Pharmaceutical Co., Osaka, Japan) were applied for 2 weeks.
Statistical analysis
Ocular parameters such as 'IOP', 'visual acuity (LogMAR)' and 'AC depth' before and after surgery were recorded at each visit. One-way analysis of variance (SPSS, Inc., Chicago, IL, USA) was used for post hoc comparison between matched groups. P-values <0.05 were considered statistically significant. The remaining data were analyzed as descriptive statistics.
Results
Thirty eyes of 30 patients (8 males and 22 females) with an average age of 53.6±13.2 SD years (range: 20–78) were included in this study. The mean duration of malignant glaucoma was 8.6±2.8 days (range: 4–14). The average axial length of the eyes was 21.3±0.8 mm. The results of UBM showed a mean AC depth of 0.8±0.4 mm (range: 0.2–1.4), which demonstrated a decrease or disappearance of the AC13 (Figure 1). The characteristics of the patients are listed in Table 1. The pre-operative IOP varied from 25 mm Hg to 58 mm Hg, with a mean pressure of 34±8.3 mm Hg. The VA (LogMAR) ranged from 0.4 to 1.7. Pre-operative treatment included mydriatics, cycloplegics, topical anti-glaucoma drops, oral carbonic anhydrase inhibitors, and mannitol. In some patients these treatments temporary deepened the AC and decreased IOP, but ciliary block was not resolved.
Following our intervention, AC depth measured by UBM increased from 0.8±0.4 mm to 2.7±0.3 mm 1 week after surgery (P<0.001), and was statistically significant at 6 months (F=486.597, P<0.001; Figure 2). The mean IOP in all patients measured on the first day decreased from 34±8.3 mm Hg to 10.5±4.1 mm Hg; the difference was significant (P<0.001). This significant difference in IOP was stable over 6 months (F=66.136, P<0.001; Figure 3). Best-corrected VA improved in most patients, this improvement was significant 1 week after surgery (F=31.650, P<0.001) (Figure 4). Recorded data and statistical analysis of IOP, AC depth and VA (LogMAR) are listed in Tables 2 and 3.
On the basis of the results of the follow-up examinations at 6 months, normal IOP was maintained without topical or general treatment in 23 eyes, whereas only topical β-blockers were used in five eyes. In two eyes, control of IOP was not satisfactory even when topical and oral treatment was continued. Slip-lamp examination and gonioscopy showed a permanent iridocorneal adhesion and angle closure. A new trabeculectomy was performed in these two patients 2 months after the previous intervention and IOP was controlled at the last follow-up visit.
Other observed complications during the 6-month follow-up period included three cases of shallow AC the day after our intervention, and all patients had spontaneous remission within 1 week; 2 cases of AC fibrin exudation and one case of vitreous hemorrhage. No other severe ocular complications occurred.
Discussion
When reviewing the data retrieved from the patients in this study, it was observed that these patients all had short axial lengths, with an average axial length of 21.3±0.8 mm and an average AC depth of 0.8±0.4 mm. These measurements were similar to those observed by Wang et al14, who found that eyes with malignant glaucoma had an average axial length of 21.56±0.84 mm and an average AC depth of 0.32±0.32 mm. This anatomic abnormality could be related to ethnic differences. An epidemiological study showed that the prevalence of primary angle-closure glaucoma was much higher in Asia, especially in China, than in other areas.15, 16, 17, 18
The aim of malignant glaucoma therapy is to relieve the ciliolenticular block, disrupt the misdirection of aqueous humor and to restore normal aqueous flow.19 Drug therapy induces a posterior movement of the lens–iris diaphragm, reduces the production of aqueous humor, and dehydrates the vitreous volume. However, it has a low immediate success rate and the high risk of recurrence limit its application.12 Laser options are used to create a direct communication between the vitreous cavity and AC, which have resulted in a high recurrence rate in some studies.12 Laser therapy is generally not used in phakic eyes.20 Neither medication nor laser treatment can thoroughly relieve the ciliary block. Traditional surgical interventions include posterior sclerotomy, vitreous puncture, and aspiration via the pars plana and PPV.5, 21 These treatments can reduce vitreous volume and restore AC depth; but the ciliary block and aqueous misdirection still exist.22 In addition, PPV together with capsulorrhexis or capsulectomy are not enough to break the cycle of aqueous misdirection.23 In recent years, our group carried out a modified partial PPV, and the biggest difference between our surgery and the classic procedure is that only minimal vitreous behind the pre-existing peripheral iridectomy is removed by a 23-gauge vitreous cutter. In eyes with lens opacity, we found that it is not easy to position the vitrector accurately, therefore after cataract surgery we repeated the partial PPV to completely remove the vitreous body behind the peripheral iridectomy. Compared with the classic surgical procedure, the volume of removed vitreous body is smaller and the range of vitrectomy is limited, and no infusion or illumination is required. According to the theories proposed by Ng and Morgan,24 we suggest that with the removal of only a small volume of vitreous (about 0.5 ml), the vitreous body could be ‘debulked’ and the pressure added to the anterior hyaloids could be decreased. The adhesion bet ween the lens, ciliary body, and vitreous could also be relieved. In addition, following partial removal of the vitreous body, the posterior capsule would not lose the support of the vitreous cavity, which facilitated the subsequent step in our procedure.
After the first step mentioned above, phacoemulsification and IOL implantation was performed in patients without previous cataract surgery. Previous study has shown that in PPV for malignant glaucoma the success rates were higher in cases with cataract extraction.25 The main reason for this higher success rate is thought to be the guaranteed disruption of the anterior hyaloid with removal of the lens.26 In other studies,27 lens extraction was also supported due to the risk of iatrogenic cataract formation during anterior hyaloid removal. We also postulated that compared with the crystalline lens, the artificial lens which is much smaller and thinner, is less likely to push the iris and the ciliary body forward. Phacoemulsification in our patients who all had a short axial length was essential. However, there is a high risk of corneal endothelial injury and posterior capsule rupture when operating in a shallow AC. Therefore, we completed the partial PPV first to reduce the posterior vitreous pressure, which helped in the formation of the AC and resulted in a safe surgical procedure.
Following IOL implantation, to correct aqueous humor misdirection through the body–zonule–lens capsule complex, the formation of peripheral communication is pivotal. As stated in other reports, we also performed a zonulohyaloidectomy in our patients. This was performed from the back through the pars plana incision and was facilitated by implantation of the transparent artificial lens. If the crystalline lens is not removed, it is difficult to achieve the same communication without damaging the crystalline lens in phakic eyes.8 Thus, the steps for phacoemulsification and zonulohyaloidectomy were correlated with each other.
In our experience, high-speed vitrectomy is needed to avoid iatrogenic retinal tears on the ora serrata, and the speed should be fast enough and the resection volume should not be too large so that we can effectively prevent a sharp decline in IOP, which may cause surgical complications such as choroidal detachment and expulsive choroidal hemorrhage. At the beginning of surgery, aspiration of the vitreous should be performed to moderately reduce the IOP.
We believe that our modifications regarding the surgical treatment of malignant glaucoma are safe. All post-operative complications were treated and the patients achieved remission. In two eyes, the IOP was elevated despite our intervention. This was due to persistent goniosynechiae which was proved by gonioscopy and UBM. We speculate that persistent goniosynechiae was caused by contact between the iris and cornea for a long period of time and the chronic inflammatory stimuli produced by long-term high IOP. These patients underwent a new trabeculectomy to further create a filtering bleb.
In summary, our retrospective study showed that a modified partial PPV behind the iridectomy combined with phacoemulsification, IOL implantation and zonulohyaloidectomy was a stable procedure with the same success rate as the classic anterior vitrectomy. ACs were formed and the post-operative IOPs were well controlled with limited complications. Due to the safety and simplicity of this intervention, we believe that this is an attractive therapeutic option for surgeons treating patients with malignant glaucoma who fail to respond to routine medication/laser treatment. New studies with longer follow-up and a larger sample size will be carried out to confirm the safety and efficiency of this intervention.

References
Dumitrica DM, Stefan C . Malignant glaucoma. Oftalmologia 2007; 51 (3): 327.
Luntz MH, Rosenblatt M . Malignant glaucoma. Surv Ophthalmol 1987; 32: 73–93.
Stumpf TH, Austin M, Bloom PA, McNaught A, Morgan JE . Transscleral cyclodiode laser photocoagulation in the treatment of aqueous misdirection syndrome. Ophthalmology 2008; 115 (11): 2058–2061.
Herschler J . Laser shrinkage of the ciliary processes. A treatment for malignant (ciliary block) glaucoma. Ophthalmology 1980; 87: 1155–1159.
Stumpf TH, Austin M, Bloom PA, McNaught A, Morgan JE . Transscleral cyclodiode laser photocoagulation in the treatment of aqueous misdirection syndrome. Ophthalmology 2008; 115 (11): 2058–2061.
Veroniek D, Peter S, Joachim VC . Outcomes of different management options for malignant glaucoma:a retrospective study. Graefes Arch Clin Exp Ophthalmol 2012; 250 (1): 131–134.
Francis BA, Wong RM, Minckler DS . Slit-lamp needle revision for aqueous misdirection after trabeculectomy. J Glaucoma 2002; 11: 183–188.
Sharma A, Sii F, Shah P, Kirkby GR . Vitrectomy-phacoemulsification-vitrectomy for the management of aqueous misdirection syndromes in phakic eyes. Ophthalmology 2006; 113 (11): 1968–1973.
Chen SD, Salmon JF, Patel CK . Videoendoscope-guided fluorescein-assisted vitrectomy for phakic malignant glaucoma. Arch Ophthalmol 2005; 123 (10): 1419–1421.
Lois N, Wong D, Groenewald C . New surgical approach in the management of pseudophakic malignant glaucoma. Ophthalmology 2001; 108 (4): 780–783.
Zhou C, Qian S, Yao J, Tang Y, Qian J, Lu Y et al. Clinical analysis of 50 chinese patients with aqueous misdirection syndrome: a retrospective hospital-based study. J Int Med Res 2012; 40: 1568–1579.
Debrouwere V, Stalmans P, Van Calster J, Spileers W, Zeyen T, Stalmans I . Outcomes of different management options for malignant glaucoma: a retrospective study. Graefes Arch Clin Exp Ophthalmol 2012; 250: 131–141.
Al-Farhan HM, Almutairi RN . Anterior segment biometry using ultrasound biomicroscopy and the Artemis-2 very high frequency ultrasound scanner. Clin Ophthalmol 2013; 7: 141–147.
Wang Z, Huang J, Lin J, Liang X, Cai X, Ge J . Quantitative measurements of the ciliary body in eyes with malignant glaucoma after trabeculectomy using ultrasound biomicroscopy. Ophthalmology 2014; 121 (4): 862–869.
Rotchford AP, Kirwan JF, Muller MA, Johnson GJ, Roux P . Temba glaucoma study: a population based crose-sectional survey in urban south Africa. Ophthalmology 2003; 110 (2): 376–382.
Yamamoto T, Iwase A, Araie M, Suzuki Y, Abe H, Shirato S et al. The Tajimi Study report 2: prevelence of primary angle closure and secondary glaucoma in the Japanese population. Ophthalmology 2005; 112 (10): 1661–1669.
He M, Foster PJ, Ge J, Huang W, Zheng Y, Friedman DS et al. Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan district, Guangzhou. Invest Ophthalmol Vis Sci 2006; 47 (7): 2782–2788.
Sakata K, Sakata LM, Sakata VM, Santini C, Hopker LM, Bernardes R et al. Prevelance of glaucoma in a South brazilian population: projeto Glaucoma. Invest Ophthalmol Vis Sci 2007; 48 (11): 4974–4979.
Muqit MMK, Mj M . Malignant glaucoma after phacoemulsification: treatment with diode laser cyclophotocoagulation. J Cataract Refract Surg 2007; 33: 130–132.
Ruben S, Tsai J, Hitchings RA . Malignant glaucoma and its management. Br J Ophthalmol 1997; 81: 163–167.
Dumont HB, Lehoux BA, Santiago PY . Le laser diode dans le traitement du glaucoma malin. J Fr Ophtalmol 2006; 29 (2): 2573–2577.
Johnson DH . Options in the management of malignant glaucoma. Arch Ophthalmol 1998; 116: 799–800.
Bitrian E, Caprioli J . Pars plana anterior vitrectomy, hyaloido-zonulectomy, and iridectomy for aqueous humor misdirection. Am J Ophthalmol 2010; 150 (1): 82–87.
Ng WT, Morgan W . Mechanisms and treatment of primary angle closure: a review. Clin Experiment Ophthalmol 2012; 40 (4): e218–e228.
Tsai JC, Barton KA, Miller MH, Khaw PT, Hitchings RA . Surgical results in malignant glaucoma refractory to medical or laser therapy. Eye 1997; 11: 677–681.
Harbour JW, Rubsamen PE, Palmberg P . Pars plana vitrectomy in the management of phakic and pseudophakic malignant glaucoma. Arch Ophthalmol 1996; 114: 1073–1078.
Cekic O, Batman C . Pars plana vitrectomy in the treatment of phakic and pseudophakic malignant glaucoma. Arch Ophthalmol 1998; 116: 118.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
He, F., Qian, Z., Lu, L. et al. Clinical efficacy of modified partial pars plana vitrectomy combined with phacoemulsification for malignant glaucoma. Eye 30, 1094–1100 (2016). https://doi.org/10.1038/eye.2016.106
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.106
This article is cited by
-
Impact of improved minimally invasive anterior vitrectomy on the prognosis of patients with malignant glaucoma
BMC Ophthalmology (2024)
-
Pars plana subcapsulotomy to remove condense subcapsular opacification in combined surgery of silicone oil removal and phacoemulsification
International Ophthalmology (2022)
-
Current Concepts on Aqueous Misdirection
Current Ophthalmology Reports (2019)
-
Surgical management of malignant glaucoma: a retrospective analysis of fifty eight eyes
Eye (2017)